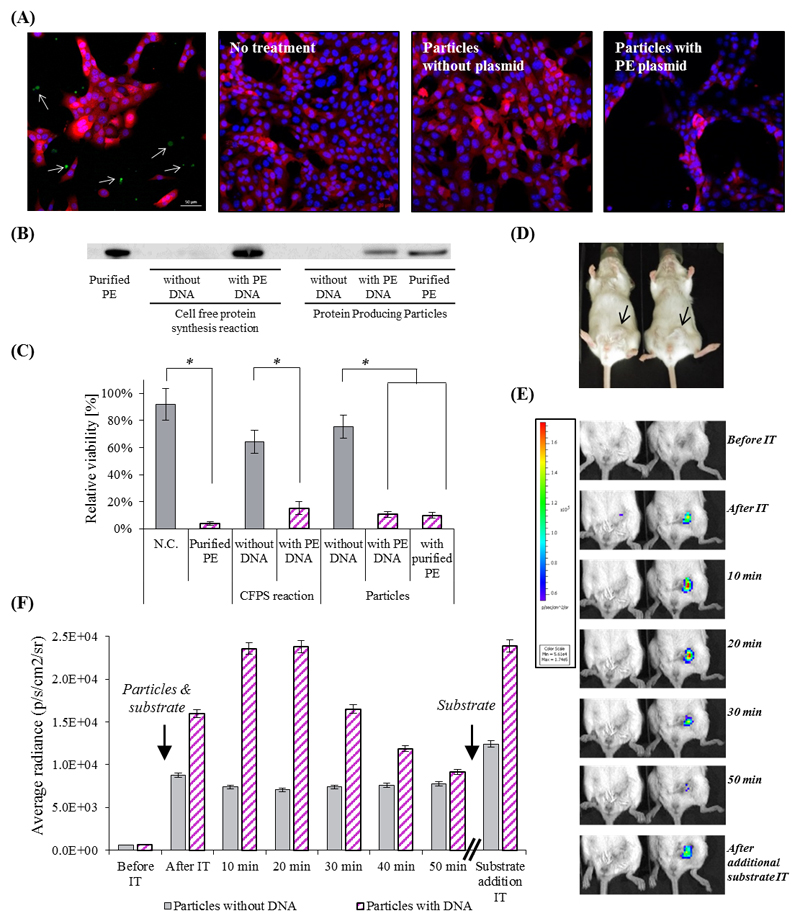
Figure 3. The therapeutic effect of artificial cell-like particles.
(A) Confocal microscopy analysis of the therapeutic potency of PE-producing particles on 4T1 breast cancer cells expressing a reporter mCherry gene. Upper left - Confocal microscopy image of mCherry cells with particles producing sfGFP. The cytoplasm of 4T1 cells is labeled red, the nucleus is labeled blue, and produced sfGFP is green (represented by arrows). Second and third to left - cells which were not treated or treated with the particles lacking a DNA template, respectively. Right – cells treated with particle containing PE-coding DNA template. (B) Western blot analysis verifies the production of PE inside the particles. (C) Cell viability was evaluated by an MTT assay. Error bars represent standard deviation of the mean from 3 independent repeats. *Significant difference between treatments, where p-value<0.05 according to a Student's t-test with a two-tailed distribution with equal variance. (D)&(E)&(F) Renilla Luciferase in vivo production inside particles injected to BALB/c mice bearing orthotopic 4T1 tumors in the mammary fat pad (D). (E) Protein producing particles DNA-encoded (right), or non-encoded (left), to synthesize luciferase, were injected intra-tumor with enzyme’s substrate. The luminescent signal from the particles was monitored using whole animal imaging for 50 min. At this point the luminescent signal decayed, and the substrate was refurbished, resuming the luminescent signal. The maximal instrument internal error is 3% of the obtained value.