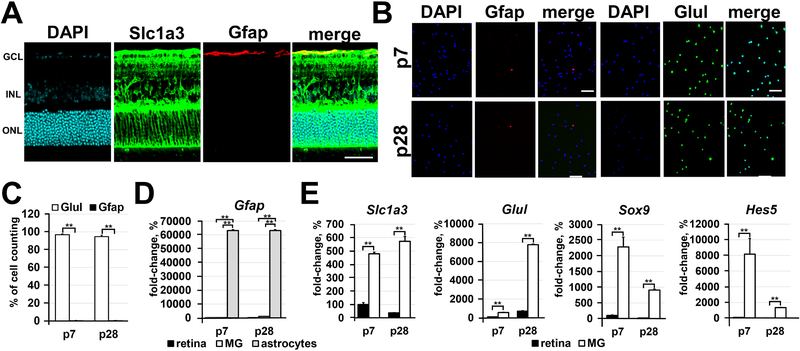
Figure 1.
Immunomagnetic separation is an effective approach for the isolation of highly pure Müller glia. A) The spatial distribution of Glast (Slc1a3) proteins evaluated by immunohistochemistry in P28 retinas reveals the presence of Glast mostly in Müller glia and, to some extent, in retinal astrocytes (labeled by Gfap). DAPI was used to visualize the cell nucleus. Bar is 50 μm. (GCL - ganglion cell layer; INL - inner nuclear layer; ONL - outer nuclear layer) B) Since the anti-Glast (Slc1a3) antibody can recognize Müller glia and some retinal astrocytes, we tested the distribution of Glast+ cells isolated from the retina depending on cell type using the Müller glia marker (Glul; glutamine synthetase) and astrocyte marker (Gfap). Glast+ cells were isolated from retinas of P7 and P28 animals. Bar is 50 μm. C) The percentage of Müller glia (Glul; glutamine synthetase marker) and astrocytes relative to the total number (DAPI labeled cells) of Glast+ counted cells was calculated. D) and E) Expression of astrocyte and Müller glia markers in the entire retina and in isolated astrocytes, P7 and P28 Glast+ cells was evaluated using qRT-PCR. Astrocytes isolated from P3 brains were used as a positive control for Gfap expression. For each gene, the results are expressed as a fold-change of the corresponding value for P7 retina ± SE of the mean (n = 6).