Abstract
BACKGROUND:
Caesarean section is one of the commonest gynaecological surgeries.
AIM:
Given the importance of pain relief after caesarean section surgery as well as contradictions in the studies conducted on intravenous lidocaine analgesic effects, this study aimed to evaluate the effect of adding lidocaine to patient-controlled analgesia (PCA) with morphine on pain intensity after caesarean section surgery.
MATERIAL AND METHODS:
In a double-blinded, randomised clinical trial, 80 women who were scheduled for caesarean section surgery with spinal anaesthesia at Sari Imam Khomeini Hospital in 2017 were randomly assigned into two intervention and control groups. After surgery, all patients were connected to a morphine PCA pump. The PCA solution (total volume = 100 ml) in intervention group contained 50 ml of 2% lidocaine and 30 mg (3 ml) of morphine in 47 ml normal saline. In the control group, the PCA pump contained 30 mg (3 ml) of morphine, and the rest (97 cc) was normal saline. Patients’ pain intensity was assessed at 2, 4, 6, 12, 18 and 24 hours after surgery using a visual analogue scale (VAS). Additionally, their postoperative nausea and vomiting, duration of hospitalisation, duration of ileus relapse after surgery, and patients’ satisfaction after surgery were evaluated. Data were analysed using SPSS version 22 software.
RESULTS:
The mean and standard deviation of pain intensity in all patients at the intervals of 2, 4, 6, 12, 18 and 24 hours after surgery were 5.91 ± 1.57, 4.97 ± 1.55, 3.84 ± 1.60, 3.54 ± 1.45, 2.56 ± 1.70 and 0.94 ± 1.70, respectively. Data analysis revealed that, regardless of the groups, postoperative pain intensity significantly decreased (P < 0.0001). However, there were no significant differences between the two groups in terms of mean postoperative pain intensity at any time interval (p > 0.05). Also, there was no significant difference between the two groups in terms of frequency of receiving the diclofenac suppositories after the surgery (p > 0.05). Additionally, there was no statistically significant difference between the two groups in terms of postoperative nausea and vomiting, duration of hospitalisation, duration of postoperative ileus relapse and patients’ satisfaction (p > 0.05).
CONCLUSION:
Based on the results of this study, it seems that adding lidocaine to PCA with morphine, compared with morphine PCA alone, do not have a significant effect on reducing the pain intensity after cesarean section using spinal anaesthesia. Although, further studies with larger sample size are warranted.
Keywords: Lidocaine, Morphine, Caesarean Section, Gynecologic Surgical Procedures, Analgesia, Patient-Controlled
Introduction
Caesarean section is one of the commonest gynaecological surgeries. Its prevalence of sectarian section is increasing due to various causes, such as increased marriage age and socioeconomic status of the community; so that 65% of Iranian pregnant women underwent cesarean section and about 22.5% of all deliveries in the world are performed by cesarean section. Thus, cesarean section surgery is one of the health priorities of the community [1]. After surgery, patients experience pain inevitably at varying levels. Following caesarian section surgery, patients suffer from acute pain due to a complex physiological response to tissue damage, visceral dilatation, and uterus contractions [2], [3]. Postoperative pain results in undesirable physiological effects, such as the lack of discharge of respiratory secretions, ileus, and prolonged bed rest, leading to increased risk of deep vein thrombosis and delay in onset of breastfeeding. Therefore, finding a way to minimise complications and bring the highest pain relief for the patient is one of the most important issues after the cesarean section surgery [4], [5]. Opioids, especially in their injectable forms, are highly used to relieve acute postoperative pain, including cesarean section [4], [6]. Moreover, pain is a multifactorial phenomenon which is not completely controlled merely by opioids. Additionally, using opioids is associated with dose-related complications such as respiratory depression, nausea, vomiting, urinary retention, itching, drowsiness or ileus [7].
Thus, it seems reasonable to use compounds that can mitigate the pain intensity in the patients without causing side effects caused by using opioids. Lidocaine is one of the drugs used to relieve pain after surgery. When the use of opioids causes insufficient pain relief or excessive side effects, the use of lidocaine is an appropriate option to control visceral and central pains [8]. Various studies have used lidocaine for postoperative pain control, which has led to the controversial results [9], [10], [11], [12], [13], [14]. Also, doing further studies to investigate the effects of intravenous lidocaine in controlling acute postoperative pain in various surgical procedures have been recommended [15]. It has been previously confirmed that the excretion of lidocaine in breast milk is low and the potential disadvantages caused by child breastfeeding of a mother who has received lidocaine is negligible [16], [17]. Therefore, considered to the abovementioned issues and the importance of pain control after cesarean section surgery, as well as the contradictions in the literature regarding the effect of intravenous lidocaine, as an adjuvant for postoperative pain management, this study was conducted aiming to evaluate the effect of adding lidocaine to patient-controlled analgesia (PCA) with morphine on pain intensity after caesarean section surgery.
Material and Methods
After obtaining approval from the institutional Ethics Committee and informed written consent from patients, 80 women who were scheduled for caesarean section surgery with spinal anaesthesia at Sari Imam Khomeini Hospital were included in this double-blind, randomised, clinical trial. The study was carried out between October and December 2018 and registered in the Iranian Registry of Clinical Trials Database (IRCT20100713004365N23).
The inclusion criteria were candidate for non-emergency cesarean section under the spinal anaesthesia, age between 20 and 45 years, American Society of Anesthesiologists (ASA) class I, first or second pregnancy, lack of sensitivity to lidocaine or bupivacaine, no previous intra-abdominal surgery, lack of multiple pregnancy, lack of preoperative pain, lack of substance abuse and psychotropic drugs uses, and body mass index (BMI) less than 40. Exclusion criteria were the patient’s unwillingness to continue studying at any time, midline surgical incision for any cause, the occurrence of any unusual complications during surgery and lack of willingness to perform spinal anaesthesia. Of all patients who evaluated, 80 patients met the inclusion criteria and randomly allocated into two groups using computer-generated random numbers. This study was double-blinded study, in which the statistician, the project executor and the patients were unaware of the assignment of patients in the treatment groups, and the data collection form was completed by a project executor collaborator who was unaware of the study groups. Before the surgery, patients’ demographic characteristics such as age, educational level, weight (kg), height (cm), duration of surgery, getting out of bed after surgery were also evaluated and recorded. Detailed explanations were provided on the way of measuring the severity of postoperative pain and nausea and vomiting using the Visual Analog Scale (VAS) as well as the use of the PCA pump, and the necessary training was provided in this regard. Before the anaesthetic procedure, a good peripheral vein was taken from a non-dominant hand. Thirty minutes before the procedure, 7 ml/kg of crystalloid serum was given to these patients, and then spinal anaesthesia was performed. Patients were placed in the sitting position, and spinal punctures were performed in the L2-L4 and L4-L5 space using Quinke G25 spinal needle and midline approach.
The PCA pump in the intervention group contained 50 ml of lidocaine 2% plus 30 mg of morphine (3 ml) and the rest was 47 cc normal saline (total volume of the pump was 100 ml). In the control group, the PCA pump contained 30 mg of morphine (3 ml), and the rest (97 cc) was normal saline. The pumps were numbered 1 and 2 and were similar in terms of volume and appearance, and only the nurse who was the project contributor was aware of it, and other staffs including surgeon and anesthesiologist were not aware of the two groups. After completion of anaesthesia and recovery of patients, all patients were connected to a morphine PCA pump. The settings for the PCA pump were as follows: bolus dose of 0.5 cc and 15 minutes of lockout interval and background infusion rate of 2 cc/h.
The primary outcome of this study was the postoperative pain intensity that was assessed by a nurse using VAS at times of 2, 4, 6, 12, 18, 24 hours after surgery when the patient was at the resting position. The secondary outcomes were patient satisfaction from pain control, postoperative nausea and vomiting, duration of patient hospitalisation, and the duration of ileus relapse after surgery.
Statistical Analysis
Data were analysed by using SPSS software version 22. Frequency and percentage were used to show qualitative variables and mean, and the standard deviation was used to show the quantitative variables. To examine the correlation between quantitative variables, the Pearson or Spearman coefficients were calculated, and Chi-Square test was used to examine the relationship between qualitative variables. The P value < 0.05 was considered as significant level.
Results
A total of 91 consecutive patients were screened during the study period. Of these, 8 patients did not meet the inclusion criteria, and 3 patients declined to participate in the study. The remaining 80 patients were randomly allocated to two equal-sized groups (Figure 1).
Figure 1.
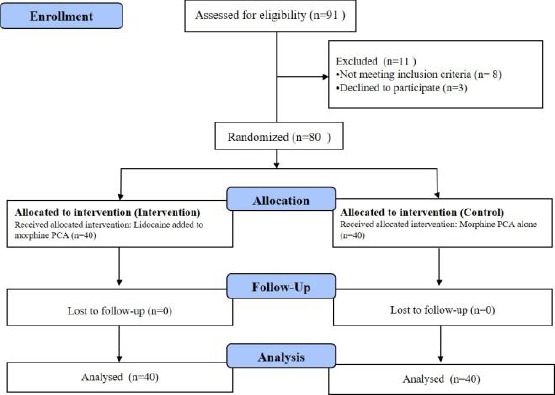
Flow chart of the study
The mean age of patients was 29.83 ± 3.65 years. 92.5% and 100% of the women in the intervention and control groups had a diploma degree, respectively. A total of 20% of the subjects were a candidate for the first cesarean section with spinal anaesthesia, 43.8% of whom had 1 child, 33.8% had two children, and 2.5% had 3 children (p > 0.05). The mean BMI of the subjects was 31.18 ± 3.91 and 30.93 ± 3.24 in the intervention and control groups, respectively (p = 0.487). The mean duration of surgery in the intervention group was 63.6 ± 10.4 and 65.75 ± 7.8 minutes in the control group. There was no statistically significant difference between the two groups in this regard (P = 0.301). The duration of ileus relapse was 18.73 ± 3.73 hours in the intervention group and 18.87 ± 4.29 hours in the control group, which was not statistically significant between the two groups (p = 0.868). The mean duration of hospitalisation in the control group was slightly higher than that in the intervention group, although there was no significant difference between the two groups (1.90 ± 0.30 days in the control group and 1.82 ± 0.38 days in the intervention group, p = 0.336). The mean and standard deviation of the pain intensity of the patients in the two groups at the specified intervals of 2, 4, 6, 12, 18, and 24 hours after the surgery was 5.91 ± 1.57, 4.97 ± 1.55, 3.84 ± 1.60, 3.54 ± 1.45, 2.56 ± 1.70 and 0.94 ± 1.70, respectively (Figure 2). The trend of the changes was statistically significant (P < 0.0001).
Figure 2.
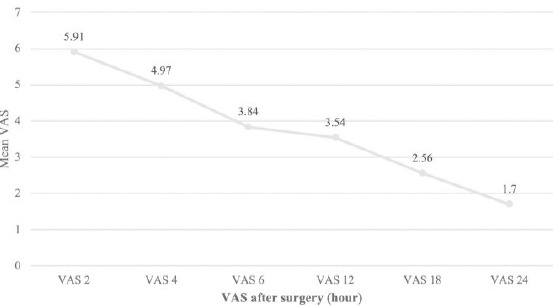
The mean VAS pain intensity score at specified intervals after the surgery in all patients
The mean pain intensity of patients after the surgery at specified intervals in both intervention and control groups is shown in Table 1 and Figure 1.
Table 1.
The mean VAS pain intensity score at specified intervals of 2, 4, 6, 12, 18, and 24 hours after surgery in two groups
| Variable | Intervention group | Control group | P value |
|---|---|---|---|
| VAS 2 (Pain severity 2 hours after the surgery) | 5.75 ± 1.67 | 6.08 ± 1.47 | P = 0.360 |
| VAS 4 (Pain severity 4 hours after the surgery) | 5.07 ± 1.55 | 4.87 ± 1.55 | P = 0.567 |
| VAS 6 (Pain severity 6 hours after the surgery) | 3.93 ± 1.67 | 3.75 ± 1.54 | P = 0.628 |
| VAS 12 (Pain severity 12 hours after the surgery) | 3.60 ± 1.44 | 3.48 ± 1.48 | P = 0.704 |
| VAS 18 (Pain severity 18 hours after the surgery) | 2.65 ± 1.35 | 2.48 ± 1.21 | P = 0.545 |
| VAS 24 (Pain severity 24 hours after the surgery) | 1.85 ± 0.94 | 1.55 ± 0.94 | P = 0.158 |
As seen, there was no significant difference between the two groups in terms of pain intensity at the first 24 hours after surgery in any of the studied periods.
Figure 3.
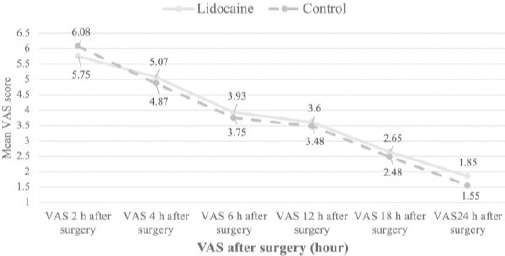
The mean pain severity based on the VAS criterion at specified intervals of 2, 4, 6, 12, 18, and 24 hours after surgery in intervention and control groups
The results of the study showed that there was no significant difference between the two groups in terms of the frequency of receiving diclofenac suppositories after the surgery (Table 2).
Table 2.
The number of frequencies of receiving diclofenac suppositories in all of the patients in the intervention and control groups
| Frequency of receiving diclofenac suppositories | 0 | 1 | 2 | 3 | 4 | P-value |
|---|---|---|---|---|---|---|
| Intervention group | 7 (%17.5) | 10 (%25) | 11 (%27.5) | 11 (%27.5) | 1 (%2.5) | P = 0.756 |
| Control group | 5 (%22.5) | 9 (%22.5) | 16 (%40) | 9 (%22.5) | 1 (%2.5) |
In the control group, none of the patients reported postoperative nausea and vomiting. In the intervention group, only one patient reported nausea and vomiting (2.5%), which was resolved after 6 hours. All patients in both groups had 100% satisfaction with cesarean section.
Discussion
The results of this study revealed that the pain intensity after the cesarean section was significantly decreased in both groups before and after 24 hours after the surgery, and the severity of postoperative pain in all hours in both intervention and control groups decreased significantly compared to the first hour of pain. In general, the pain severity in the first 6 hours after surgery in the intervention group was lower than that in the control group. However, there was no significant difference between the two groups in terms of pain intensity at first 24 hours after surgery in any of the studied intervals. Choi et al. examined the effects of lidocaine infusion on postoperative pain intensity after breast surgery. For patients in the intervention group, about thirty minutes before the incision, lidocaine was injected at a dose of 1.5 mg/kg. Then, lidocaine infusion with a dose of 1.5 mg/kg was performed up to incision closure. The results showed that intravenous lidocaine had no significant effect on postoperative pain severity in patients [13]. Also, another study in patients undergoing hip arthroplasty showed that continuous infusion of low dose lidocaine (1.5 mg/kg) compared to placebo, did not affect the pain intensity and reducing the opioid consumption after surgery [9]. The results of Moslemi et al. on patients undergoing gynecologic laparoscopic surgery indicate that infusion of lidocaine (1.5 mg/kg) could significantly affect postoperative pain reduction [18]. Also, it has been shown that adding lidocaine to morphine PCA, compared to morphine PCA alone, has no significant efficacy in postoperative pain reduction after abdominal surgery [19]. The results of these studies are consistent with our study. In a study conducted by Alebouyeh et al, the effects of adding lidocaine to morphine in three groups of lidocaine 1% with 10 mg of morphine, 1% lidocaine with 20 mg of morphine and the control group with just 20 mg of morphine showed that the mean pain intensity decreased significantly in the groups in which lidocaine was added to morphine [12]. Choi et al., evaluate the effects of intravenous lidocaine infusion on the duration of the ileus and duration of hospitalisation and the severity of postoperative pain. The results revealed that the use of lidocaine had a significant effect on the narcotic drug consumed, intestinal function and duration of hospitalisation [13]. Another study showed that the administration of lidocaine (bolus dose and then infusion dose) 30 minutes before surgery for up to 1 hour after surgery led a reduction in duration of hospitalisation [20]. Also, it has been revealed that infusion of adding lidocaine, at a dose of 1.5 mg/kg, to morphine which began 30 minutes before the surgical incision and continued until 1 hour after the completion of the surgery, had significant effect on reduction of pain intensity and postoperative morphine consumption after major abdominal surgery [10]. Yardeni et al. showed a significant effect of intravenous lidocaine administration before surgery (2 mg/kg), and its infusion during surgery (1.5 mg/kg) incision on the reduction of pain intensity in patients undergoing abdominal hysterectomy [11]. In a study conducted by Groudine et al with the aim of evaluating the effectiveness of intravenous lidocaine on the function of intestinal movements, postoperative pain, duration of hospitalization in patients underwent prostatectomy, showed that bolus administration of lidocaine (1.5 mg/kg) and then its infusion (3 mg/min), led to early back of intestines function and reduced postoperative pain [20]. The results of the mentioned research were different from those of our study in terms of comparing the pain intensity in adding lidocaine on postoperative pain intensity. Khoshrang et al., evaluated the effectiveness of 0.25% marcain injection plus lidocaine 2% in the surgical site on the severity of pain in patients undergoing cesarean section with spinal anaesthesia. The results showed that topical injection of marcain (0.25%), compared to lidocaine, in the cesarean section could decrease pain severity in the early hours after surgery and also reduce the need for analgesics [21].
In conclusion, according to the research results, it seems that adding lidocaine to PCA morphine, compared to morphine PCA alone, did not have a significant effect on reducing the postoperative pain intensity after cesarean section with spinal anaesthesia.
Footnotes
Funding: This research has been financially supported by the deputy of research and technology, Mazandaran University of Medical Sciences, Sari, Iran
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Rafiei M, Saei Ghare M, Akbari M, Kiani F, Sayehmiri F, Sayehmiri K, et al. Prevalence, causes, and complications of cesarean delivery in Iran:A systematic review and meta-analysis. Int J Reprod Biomed (Yazd) 2018;16(4):221–234. https://doi.org/10.29252/ijrm.16.4.221. [PMC free article] [PubMed] [Google Scholar]
- 2.Kintu A, Abdulla S, Lubikire A, Nabukenya MT, Igaga E, Bulamba F, et al. Postoperative pain after cesarean section:assessment and management in a tertiary hospital in a low-income country. BMC Health Serv Res. 2019;19(1):68. doi: 10.1186/s12913-019-3911-x. https://doi.org/10.1186/s12913-019-3911-x PMid:30683083 PMCid:PMC6347795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gholipour Baradari A, Firouzian A, Hasanzadeh Kiabi F, Emami Zeydi A, Khademloo M, Nazari Z, et al. Bolus administration of intravenous lidocaine reduces pain after an elective caesarean section:Findings from a randomised, double-blind, placebo-controlled trial. J Obstet Gynaecol. 2017;37(5):566–570. doi: 10.1080/01443615.2016.1264071. https://doi.org/10.1080/01443615.2016.1264071 PMid:28604179. [DOI] [PubMed] [Google Scholar]
- 4.Lavand'homme P. Postoperative cesarean pain:real but is it preventable? Curr Opin Anaesthesiol. 2018;31(3):262–267. doi: 10.1097/ACO.0000000000000585. https://doi.org/10.1097/ACO.0000000000000585 PMid:29521684. [DOI] [PubMed] [Google Scholar]
- 5.Unlugenc H, Vardar MA, Tetiker S. A comparative study of the analgesic effect of patient-controlled morphine, pethidine, and tramadol for postoperative pain management after abdominal hysterectomy. Anesth Analg. 2008;106(1):309–12. doi: 10.1213/01.ane.0000287815.32869.2a. https://doi.org/10.1213/01.ane.0000287815.32869.2a PMid:18165596. [DOI] [PubMed] [Google Scholar]
- 6.Hasanzadeh Kiabi F, Soleimani A, Habibi MR, Emami Zeydi A. Can vitamin C be used as an adjuvant for managing postoperative pain?A short literature review. Korean J Pain. 2013;26(2):209–10. doi: 10.3344/kjp.2013.26.2.209. https://doi.org/10.3344/kjp.2013.26.2.209 PMid:23614091 PMCid:PMC3629356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pogatzki-Zahn EM, Segelcke D, Schug SA. Postoperative pain-from mechanisms to treatment. Pain Rep. 2017;2(2):e588. doi: 10.1097/PR9.0000000000000588. https://doi.org/10.1097/PR9.0000000000000588 PMid:29392204 PMCid:PMC5770176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firouzian A, Alipour A, Rashidian Dezfouli H, Zamani Kiasari A, Gholipour Baradari A, Emami Zeydi A, et al. Does lidocaine as an adjuvant to morphine improve pain relief in patients presenting to the ED with acute renal colic?A double-blind, randomized controlled trial. Am J Emerg Med. 2016;34(3):443–8. doi: 10.1016/j.ajem.2015.11.062. https://doi.org/10.1016/j.ajem.2015.11.062 PMid:26704774. [DOI] [PubMed] [Google Scholar]
- 9.Martin F, Cherif K, Gentili ME, Enel D, Abe E, Alvarez JC, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008;109(1):118–23. doi: 10.1097/ALN.0b013e31817b5a9b. https://doi.org/10.1097/ALN.0b013e31817b5a9b PMid:18580181 PMCid:PMC2728117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koppert W, Weigand M, Neumann F, Sittl R, Schuettler J, Schmelz M, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98(4):1050–5. doi: 10.1213/01.ANE.0000104582.71710.EE. https://doi.org/10.1213/01.ANE.0000104582.71710.EE PMid:15041597. [DOI] [PubMed] [Google Scholar]
- 11.Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg. 2009;109(5):1464–9. doi: 10.1213/ANE.0b013e3181bab1bd. https://doi.org/10.1213/ANE.0b013e3181bab1bd PMid:19843784. [DOI] [PubMed] [Google Scholar]
- 12.Alebouyeh MR, Imani F, Rahimzadeh P, Entezary SR, Faiz SH, Soraya P. Analgesic effects of adding lidocaine to morphine pumps after orthopedic surgeries. J Res Med Sci. 2014;19(2):122–7. [PMC free article] [PubMed] [Google Scholar]
- 13.Choi SJ, Kim MH, Jeong HY, Lee JJ. Effect of intraoperative lidocaine on anesthetic consumption, and bowel function, pain intensity, analgesic consumption and hospital stay after breast surgery. Korean J Anesthesiol. 2012;62(5):429–34. doi: 10.4097/kjae.2012.62.5.429. https://doi.org/10.4097/kjae.2012.62.5.429 PMid:22679539 PMCid:PMC3366309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuethrich PY, Romero J, Burkhard FC, Curatolo M. No benefit from perioperative intravenous lidocaine in laparoscopic renal surgery:a randomised, placebo-controlled study. Eur J Anaesthesiol. 2012;29(11):537–43. doi: 10.1097/EJA.0b013e328356bad6. https://doi.org/10.1097/EJA.0b013e328356bad6 PMid:22907609. [DOI] [PubMed] [Google Scholar]
- 15.de Oliveira CM, Issy AM, Sakata RK. Intraoperative intravenous lidocaine. Rev Bras Anestesiol. 2010;60(3):325–33. doi: 10.1016/S0034-7094(10)70041-6. https://doi.org/10.1016/S0034-7094(10)70041-6. [DOI] [PubMed] [Google Scholar]
- 16.Zeisler JA, Gaarder TD, De Mesquita SA. Lidocaine excretion in breast milk. Drug Intell Clin Pharm. 1986;20(9):691–3. doi: 10.1177/106002808602000913. https://doi.org/10.1177/106002808602000913 PMid:3757781. [DOI] [PubMed] [Google Scholar]
- 17.Sachs HC Committee On Drugs. The transfer of drugs and therapeutics into human breast milk:an update on selected topics. Pediatrics. 2013;132(3):e796–809. doi: 10.1542/peds.2013-1985. https://doi.org/10.1542/peds.2013-1985 PMid:23979084. [DOI] [PubMed] [Google Scholar]
- 18.Moslemi F, Rasooli S, Dehdilani M, Sadeghi-Bazargani H, Dehdilani M. The Effect of Intravenous Infusion of Lidocaine to Control Postoperative Pain After Gynecologic Laparoscopic Surgery:A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Iran Red Crescent Med J. 2018;20(7):e14531. https://doi.org/10.5812/ircmj.14531. [Google Scholar]
- 19.Cepeda MS, Delgado M, Ponce M, Cruz CA, Carr DB. Equivalent outcomes during postoperative patient-controlled intravenous analgesia with lidocaine plus morphine versus morphine alone. Anesth Analg. 1996;83(1):102–6. doi: 10.1097/00000539-199607000-00018. https://doi.org/10.1213/00000539-199607000-00018 PMid:8659717. [DOI] [PubMed] [Google Scholar]
- 20.Groudine SB, Fisher HA, Kaufman RP Jr, Patel MK, Wilkins LJ, Mehta SA, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86(2):235–9. doi: 10.1097/00000539-199802000-00003. https://doi.org/10.1213/00000539-199802000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Khoshrang H. Comparing the effects of local infiltration of lidocaine 2% and marcaine 0.25% on the severity of pain in patients after cesarean section. Jour Guilan Uni Med Sci. 2012;21(83):86–94. [Google Scholar]


