Abstract
Uridine 5′-triphosphate (UTP) exerts a positive inotropic effect (PIE) in isolated electrically driven isolated right atrial trabeculae carneae from patients undergoing heart surgery. This review discusses some aspects of the current knowledge on the putative receptor(s) involved and the potential biochemical transduction steps leading to the PIE.
Keywords: Utp, Inotropy, Human heart, Biochemistry, Pharmacology, P2Y-Receptors, P2X-receptors, ERK, MAPK
All mammalian cells contain uridine 5′-triphosphate (UTP), a pyrimidine derivate. UTP is essential to form RNA and is therefore crucial for cellular function [1]. UTP can, at least under some conditions, stimulate the degradation of poly(A)+RNAs, UTP can stimulate enzymes directly involved in poly(A)+RNA, or formation of uridine cyclic phosphate at the 3′ end of the RNAs [2]. In several mammalian species (Table 1, Fig. 1), UTP like ATP exerts substantial concentration- and time-dependent positive inotropic effects (PIE), notably in the human atrium where uridine 5′-diphosphate UDP is nearly ineffective (Fig. 1). While ATP has a biphasic effect (suggesting the activation of two different, but presently unknown receptors) on force of contraction in human atrium, an initial decline in force followed by a pronounced PIE, UTP exerts hardly any initial negative inotropic effect in human atrium but only a sustained positive inotropic effect (Fig. 1). The time course of the PIE of UTP is much slower than the time course of the PIE of β-adrenoceptor agonists in the human atrium (Fig. 1): this is one piece of evidence that different mechanisms are operative for the PIE of UTP compared to β-adrenergic stimulation in cardiomyocytes. In isolated right atrial preparations or living animals, UTP did not increase the rate of the heart beat (mouse: [3]). The PIE of UTP could be of clinical interest: the content of UTP in the extracellular space can be altered and thus might actively modulate contractility. UTP can be degraded by enzymatic (using for instance the enzyme CD39) break down on the surface of cardiovascular cells (Fig. 2) or by enzymes present in the plasma [4] to UDP, UMP, uridine, pyrophosphate or phosphate. For instance, the concentrations of ATP (UTP levels were no reported and thus would be a valuable goal of a further study), were increased after application of the CD39 inhibitor polyoxotungstate (Na6[H2W12O40], (see Fig. 2) [5]. Interestingly, under normal conditions CD39 was mainly expressed on endothelial cells, but after myocardial infarction CD39 was also detected on the surface of cardiomyocytes using immunohistochemistry [5]. The extracellular concentration of UTP is in the range of 10–100 nM. Conceivably, drugs might be given to patients that inhibit the degradation of UTP in the plasma or on cell membranes in the heart and this would be expected to lead indirectly to a PIE in the heart by increasing UTP levels in the circulation and hence in the vicinity of sarcolemmal receptors [5]. Vascular effects of UTP have to be considered: depending upon the species tested, the anatomic location of the vessel (coronary versus peripheral vessel) and even the prevalent disease state of the vessel investigated, both vasoconstrictory and vasodilatory effects of UTP have been reported [6, 7, 8]. This is an important issue for any drug with a PIE. If the drug induced a vasoconstriction of coronary arteries, cardiac ischemia would be induced and the supply of oxygen for the heart would be diminished and heart failure would be worsened. Likewise, vasoconstriction of peripheral resistance vessels would induce hypertension which would also be detrimental for cardiac function because more cardiac output would be required and hence the oxygen demand of the heart would increase and hence heart failure would be expected to occur. For instance, infusion of UTP in patients in arterial vessels (forearm, nine healthy volunteers, mean age 27.2 years) led to vasodilatation (6). In vitro in porcine (six months old, mechanically removed endothelium) and human coronary arteries (heart transplant recipients, endothelium removed with Trion-X-100) UTP and moreover a metabolically stable derivate of UTP (UTPγS) induced vasoconstriction with a EC50-value of 30 μM [7, 8]. There seems, however, to be a role for the endothelium in human coronary arteries. If the endothelium is functionally intact, then UTP induces vasodilation in isolated human coronary arteries [9] and presumably in patients. Hence, it is probably not feasible to treat patients with heart failure as a consequence of coronary heart disease with UTP as a PIE, because in these patients UTP would induce vasoconstriction of the coronary arteries. However, if the endothelium were intact (patients with idiopathic cardiomyopathy that is without involvement of coronary arteries) UTP might be a useful inotrope. The advantage to current inotropes like β-adrenoceptor agonists (e.g. dobutamine) or phosphodiesterase inhibitors (e.g. milrinone) come from the fact that UTP increase force of contraction without elevating cyclic AMP levels which is quite likely to induce cardiac arrhythmias.
Table 1.
Species dependent cardiac effects of UTP, ATP, Adenosine.
| Species/tissue | UTP | ATP | Adenosine |
|---|---|---|---|
| Human atrium | PIE, antiadrenergic: [37] | Biphasic: NIE, PIE: [38] |
Biphasic: NIE, PIE [39, 40, 41] |
| Human ventricle | n.d. | n.d. | No effect alone Antiadrenergic: [29, 30] |
| Rat atrium | PIE: [42] | NIE, PIE: [42] NIE, PIE: biphasic [43] PIE: [44] |
NIE, NCE [45, 46] |
| Rat ventricle | PIE: [47] NIE: [43] |
NIE: [45] | |
| Neonatal rat cardiomyocytes | PIE, PCE: [22] | ||
| Guinea pig atrium | PIE: [48] | NIE, NCE [49, 50] |
|
| Guinea pig ventricle | [50] | ||
| Mouse atrium | PIE, no NIE | PIE, NCE | |
| Mouse ventricle | PIE: [37] | PIE: [47] | |
| Chicken ventricle | PIE: [51] | PIE: [51] |
PIE = positive inotropic effect: NIE = negative inotropic effect, NCE: negative chronotropic effect, PCE = positive chronotropic effect. Overview of the contractile effects of UTP, ATP and adenosine in several mammalian cardiac preparations. Species and regional differences are apparent.
Fig. 1.
Typical effects (original tracings) of UTP, UDP, ATP and ADP (100 μM, each) on force of contraction (ordinates) in isolated electrically driven (1 Hz) trabeculae carneae from patients undergoing bypass surgery (human atrium) or wild type mouse left atrium (mouse atrium). Numbers indicate date of experiment. Time scale indicated in the abscissae. Ordinates give force of contraction. ATP elicited biphasic inotropic effects; ADP solely transient negative inotropic effect, UTP a pronounced monophasic positive inotropic effect (PIE) and UDP induced a minor PIE (unpublished original observations). This study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and has been approved by the ethics committee of the medical faculty of the Martin Luther University Halle-Wittenberg (hmbü 04.08.2005) and patients gave informed consent.
Fig. 2.
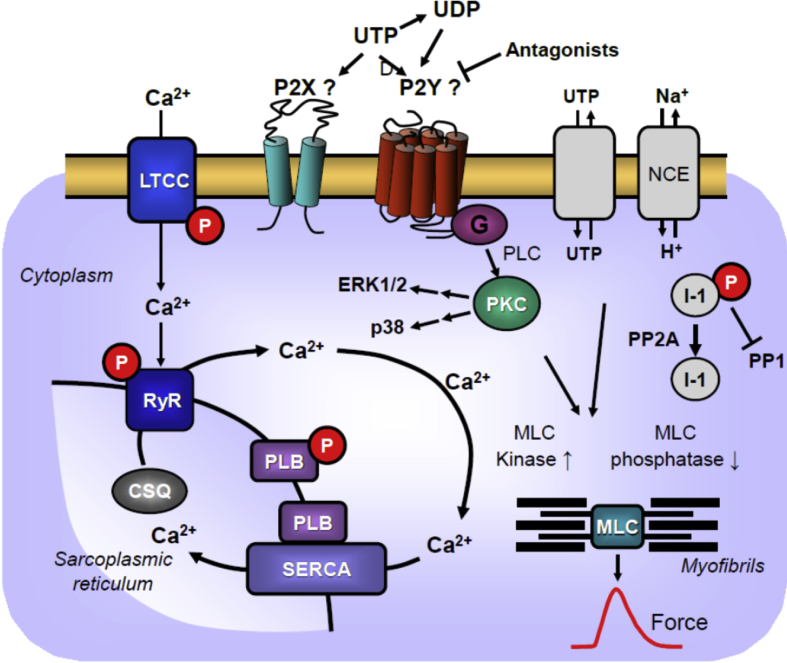
Scheme: Putative mechanism(s) of the positive inotropic effect of UTP. UTP might act on P2X- or P2Y-receptors in human cardiomyocytes. UTP can be converted by ecto-nucleoside triphosphate diphosphohydrolases (CD39) to less active UDP. Antagonists might be able to differentiate between actions on these P-receptors. In addition, UTP might be transported in both directions through proteins in the sarcolemma. UTP possibly via P2Y- or P2X-receptor stimulation can transiently enhance the phosphorylation state and activity of ERK1/2 [17] and p38, which might explain the initial PIE of UTP. These phosphorylations might also increase the current through the L-type Ca2+ channel (LTCC) and/or release of Ca2+ from the sarcoplasmic reticulum (SR) via RYR2; both processes would increase force of contraction by increasing the Ca2+ acting on myofilaments. In diastole, Ca2+ is pumped via SERCA from the cytosol into the SR. Activity of SERCA is increased by phosphorylation of phospholamban. Moreover, UTP might act via altering phosphorylation state of NCE (Na+/Ca2+ exchanger [55, 56]) to increase the Ca2+ influx into the cytosol or lower the pH of the cell. Moreover, sustained PIE of UTP might follow from an increase in the phosphorylation state of the myosin light chains (MLC) by activation of MLC kinase (following MAP kinase pathways) and/or inhibition of the activity of MLC phosphatase [57, 58]. The latter effect might follow from inhibition of PP2A (a serine/threonine phosphatase: PP) activity by MAP kinases and subsequent increased phosphorylation state and thus activation of I-1 (a specific inhibitory protein of PP1) which will lead to decreased activity of PP1. Reduced activity of PP2A [25] (and/or PP1) can increase phosphorylation of additional proteins [59] and might thus increase the Ca2+ sensitivity of myofilaments which would increase force of contraction.
In the intact outer cell membrane of cardiomyocytes and other cells types present in the human heart, there are proteins like P2X7-receptors, pannexins, connexins or anion-maxi-channels [10, 11]. These proteins might extrude UTP (like ATP which is much better investigated) out of cells but pannexins and connexins transport UTP possibly bidirectionally and thus might also transport UTP from the outside into the inside of cells: hence addition of 100 μM UTP to atrial preparations to the organ bath would generate a gradient of UTP from the outside to the inside of cardiomyocytes: however this hypothesis has to be proven or disproven experimentally by using transport inhibitory drugs (Fig. 2). The concentration of UTP in the cell is about a tenth of the concentration of ATP (around 10 mM) (rat heart: [12], rat cardiomyocytes: [13]): so physiologically there is a gradient of UTP from the inside to the outside of the cell. Whatever the mechanism(s) of release (conceivably P2X7-receptors, pannexins et cetera) might be, at least releases of UTP from endothelial cells and from cardiomyocytes have been reported: the released UTP may lead to autocrine or paracrine effects of UTP mediated by P2Y-(or P2X-) receptors located in the sarcolemma. Furthermore, it might be interesting to investigate whether P2-receptors exist in the intracellular space of cardiomyocytes. At least in rat liver, UTP can elevate Ca2+-transport into mitochondria by stimulation of functional P2-receptors (which have been identified also by immunological methods: [14]) and in addition, intracellular P2-receptors on lysozymes (in HEK-239 cells) and in nuclei (mouse liver cells) have been reported (review: [15]). If that were also the case, which needs to be elucidated, in cardiomyocytes, an interesting role for UTP acting on an intracellular signal transduction system might exist.
At least in the extracellular space a physiological regulation of the levels of UTP in the heart has been shown: upon cardiac ischemia, UTP levels in the plasma increase in pigs and humans [16]. Moreover, upon thrombin stimulation, UTP is released from erythrocytes [17]: such a release of UTP is expected to occur during myocardial infarction and might be another source of increased UTP in the vicinity of cardiomyocytes. In any case, released UTP might lead to a PIE, to cardiac protection against ischemia, tachycardia and other kinds of arrhythmia [18]. Whether UTP really causes arrhythmia or is an innocent bystander, cannot be decided from previous work: only a correlation was observed [18]: if ischemia was induced in pig hearts in vivo by occluding a coronary artery the level of UTP in the blood (in the coronary sinus) increased at times where arrhythmias occurred [18]. More work in this respect is clearly warranted to prove causality.
A role of UTP in cardiac protection is suggested by the observation that UTP pre-treatment (before induction of ischemia) of neonatal rat cardiomyocytes attenuated the ischemia induced increase in mitochondrial Ca2+-levels [19]. These effects in vitro were thought to be mediated by a degradation product of UTP namely pyrophosphate (discussed in [20]). However, pyrophosphate was ineffective in vivo, but the protection mediated by UTP was absent in P2Y−/− mice, suggesting, that the protection were mediated by this receptor. The signal transduction system of UTP (Fig. 1, Table 1) in the heart exhibits species differences. UTP, like ATP it is known to increase the phosphorylation states of MAP kinases in cardiomyocytes. For instance, UTP led to concentration- and time-dependent increases in the phosphorylation states of ERK1/2 and p38 in neonatal rat cardiomyocytes [21], adult mouse cardiomyocytes [21] and isolated human atrial preparations [22]. The increases in the phosphorylation states of ERK1/2 were transient (within 10–20 min) whereas the increases in contractile response were permanent for the time studied (at least 30 min: [22], and Fig. 1). Thus MAP kinases could be involved in the initiation of the PIE of UTP but the mechanism for the maintenance of the PIE of UTP is unknown and is suggested to result from other protein phosphorylation events. Previously, it had been shown that inhibition of p38 MAP kinase by the drug SB203580 exerted in vitro an increase in contractility [23], probably by increasing the Ca2+ sensitivity of the myofilaments [24]. More recently evidence was provided that p38 might modulate force of contraction by altering the activity of SERCA, a protein that is important for the Ca2+ homeostasis of the heart [25].
Others have also shown that the UTP-dependent protection of cardiomyocytes was independent of ERK-phosphorylation as an inhibitor of ERK phosphorylation, called U0126, did not attenuate the UTP-mediated cardiac protection against hypoxia [26]. An attractive hypothesis would be that an increase in the phosphorylation state of myosin light chains (MLC, Fig. 2) might explain the sustained PIE of UTP in the human heart.
It is difficult to say whether the PIE of UTP in human right atrium, is region specific or a general phenomenon: UTP apparently has not yet been studied with respect to a PIE in isolated preparations from the left human atrium, the right human ventricle or the left human ventricle. Such data are expected with interest. Moreover, the receptor(s) mediating the positive inotropic effect of UTP in the human heart have not yet been clearly identified. Basically, one can distinguish P1- and P-2 receptors (originally called purinoceptors, but as UTP is a pyrimidine and acts on these receptors, the abbreviation P-receptor is currently used [27]. Adenosine acts on P1-receptors which are subdivided into A1-, A2A-, A2B- and A3-receptors [28]. ATP (and UTP) act on P2-receptors, that are subdivided into P2X- and P2Y-receptors. P2X-receptors are ion channels. Their conductance are typically increased by stimulation with ATP (or possibly UTP), whereas P2Y-receptors belong to the family of G-protein coupled receptors and are usually thought to act via alteration (activation or inhibition) of adenylyl cyclase (AC) activity or phospholipase C-pathways. As seen in Table 2, there is in general a lack of potent, selective, specific antagonists for some P2-receptors, which is a limitation for pharmacology studies on the effects of UTP. As soon as newer antagonists (more potent, more selective, more specific) become available, more information on the receptor(s) involved in the PIE of UTP in the human heart should emerge. Another avenue to define the receptor(s) which mediate the PIE of UTP in the atrium, would be the study of knock out (KO) mouse models, possibly also KO mice of orphan receptors from the P2-receptor family. Some data in this regard have been produced (Table 2). However, these data have not yet positively identified which KO mouse would show a lack of UTP effects in the mouse heart. Moreover, there are receptors in the mouse which are not present in humans and vice versa. Hence, it cannot be ruled out that work on KO mouse might fail to predict which receptor is used by UTP in the human heart. Based on work from tissues other than the heart (Table 2), UTP is mainly expected to act via P2Y2-, P2Y4-, P2Y6-, P2Y11- or possibly P2Y14-receptors [27, 28, 29, 30]. However, our present data do not allow one to identify any of these receptors as the receptor that mediates the PIE of UTP in the isolated human right atrial trabeculae (Table 2), because the PIE of UTP were not antagonized by pre-incubation of the samples with typical antagonists of P2Y-receptors, that are currently available. While P2Y-receptors can act in various tissues via AC or phospholipases, using inhibitors of the activity of AC or phospholipases, we could not block the PIE of UTP in the human heart, which also argues against an action of UTP on P2Y receptors on the sarcolemma what would then translate to altered activity of AC and phospholipases in the interior of the cell [3]. However, there are species differences in the inotropic effects of UTP (see Table 1). For instance, in the mouse atrium, UTP or ATP initially exert a negative inotropic effect which is reversible over time. The reversible negative inotropic effect in mouse atrium can be regarded as the mixture of a persistent negative inotropic effect mediated by a certain receptor, the effect of which is overcome in due time by a positive inotropic effect of another receptor (Fig. 1). Some even claim that UTP might act via P2X-receptors [31], but for some P2X-receptors good antagonists are currently also not available (Table 2). Another explanation for the lack of known P-receptors antagonists to block the PIE of UTP in the human atrium is the hypothesis that these effects are mediated via orphan receptors (no antagonists available) or heteromeric P-receptors (review: [32, 33]) with incompletely understood antagonist sensitivities [34].
Table 2.
Putative receptors for UTP in the human heart.
| Receptor | Agonist | Antagonist | Human UTP effect antagonist action | UTP in KO mice |
|---|---|---|---|---|
| P2X1 | ATP | PPADS (good), suramin (good) | - | |
| P2X2 | ATP | PPADS (good), suramin (good) | - | |
| P2X3 | ATP | PPADS (good), suramin (good) | - | |
| P2X4 | ATP | PPADS (poor), | ? | |
| P2X5 | ATP | PPADS (good), suramin (good) | - | |
| P2X6 | ATP | PPADS (poor) | ? | |
| P2X7 | ATP | PPADS (poor) | ? | |
| P2Y1 | ATP | PPADS (good), RB2 (good), suramin (good) | - | |
| P2Y2 | UTP, ATP | RB2 (poor), suramin (good) AR-C1118925a |
- | - |
| P2Y4 | UTP, ATP | PPADS (poor), PSB-1454 (good) |
- | - |
| P2Y6 | UTP, UDP | PPADS (poor), suramin (poor) MRS2578 (good) |
- | - |
| P2Y11 | ATP, UTP | RB2 (good), suramin (good) | - | |
| P2Y12 | ADP | RB2 (good), suramin (good) | - | |
| P2Y13 | ADP | PPADS (poor), RB2 (good), suramin (good) | - | |
| P2Y14 | UDP-glucose | - | ||
| CystLT1R | UDP | - | ||
| CystLT2R | UDP | - |
[33, 30, 52, 53, 54]: no or negligible contractile effect in isolated electrically driven trabeculae 21, 3: carneae from human right atrium. It is apparent that presently only negative reports (-), indicating lack of identification of underlying receptor for PIE of UTP in the human heart is available. A question mark indicates that no clearcut published information is to be found in the literature.
From the literature the time course of PIE of UTP action in the human heart is comparable with that of the PIE of stimulation of α1-adrenoceptors by noradrenaline in the human heart, that is probably mediated via phosphorylation of (regulatory myosin light chain) MLC-2 [35]. One would test the assumption that UTP acts via the latter mechanism by studying whether the PIE of UTP in human atrium is accompanied by MLC-2 phosphorylation, but that has not yet been reported (Fig. 2). We speculate that UTP in intact form might enter the cardiomyocyte: this might be thermodynamically conceivable if there were local compartments of UTP. In other words, UTP was initially measured in homogenized cardiomyocytes [13]. However, we know now that for instance local concentrations can be high and cytosolic near the sarcolemma can be low: the accepted paradigms for the existence of intracellular compartments include Ca2+ and cyclic AMP. Hence, we suggest it might be an interesting avenue to synthesize UTP sensitive fluorescent dyes and stain cardiomyocytes to ascertain whether compartments of UTP in the cell exist (or not). Conceivably, intracellular UTP (if it would enter from the outside of the cell) might be used by MLC kinase to phosphorylate MLC-2 and thus increase its phosphorylation state (and thus force) or pyrophosphate (a degradation product of UTP) might inhibit MLC phosphatase, increase the phosphorylation state of MLC-2 (and thus force: compare Fig. 2). One could alternatively study whether the PIE of UTP were blocked by myosin kinase inhibitors which have been shown to block the PIE of α1-adrenergic receptors in the heart [35], or whether the PIE of UTP is enhanced in the presence of inhibitors of the activity of MLC phosphatase, for instance a Rho-associated kinase leads to MLC phosphorylation by inactivating myosin phosphatase. Fittingly, the Rho-kinase inhibitor (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclo-hexanecarboxamide (Y-27632, 50 μM) blocked the effect of PIE of α1-adrenergic receptors in the heart [34]. However, this approach has also its limitation because typical MLC kinase inhibitors like 1-(5-chloronaphthalene-1-sulfonyl)1H-hexahydro-1,4-diazepine (ML-9) at the concentrations (10–50 μM) usually used, will also inhibit cAMP- and cGMP-dependent protein kinases and might thus not lead to conclusive findings [36]. Interestingly, ERK kinase inhibitors like PD-98059 (10 μM) and SB-203580 (10 μM) did not reduce the PIE of α1-adrenergic receptors in the heart [35] which is opposite to our finding that the PIE of UTP was attenuated by the ERK inhibitor U0126 [21], possibly suggesting that the PIE of α1-adrenergic receptors and UTP in the heart do not use identical signal transduction pathways [35].
Other known positive inotropic mechanisms (which are used by ATP in the heart) like an increase in intracellular pH and increased Ca2+-transients in the cytosol [32] could be measured after stimulation of human atrial myocytes with UTP. In summary, there is a PIE of UTP in the human heart. However, the receptor(s) involved and exact signal transduction mechanism(s) for the PIE remain to be elucidated.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Anderson C.M., Parkinson F.E. Potential signalling roles for UTP and UDP: sources, regulation and release of uracil nucleotides. Trends Pharmacol. Sci. 1997;18:387–392. doi: 10.1016/s0165-6147(97)01106-1. [DOI] [PubMed] [Google Scholar]
- 2.Militello K.T., Read L.K. UTP-dependent and -independent pathways of mRNA turnover in Trypanosoma brucei mitochondria. Mol. Cell. Biol. 2000 Apr;20(7):2308–2316. doi: 10.1128/mcb.20.7.2308-2316.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gergs U., Simm A., Silber E., Neumann J. A positive inotropic effect of UTP in the human cardiac atrium. Eur. J. Pharmacol. 2014;724:24–30. doi: 10.1016/j.ejphar.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson K.A., Paoletta S., Katritch V., Wu B., Gao Z.G., Zhao Q., Stevens R.S., Kiselev E. Nucleotides acting at P2Y receptors: connecting structure and function. Mol. Pharmacol. 2015;88:220–230. doi: 10.1124/mol.114.095711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Köhler D., Eckle T., Faigle M., Grenz A., Mittelbronn M., Laucher S., Hart M.L., Robson S.C., Müller C.E., Eltzschig H.K. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007 Oct 16;116(16):1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 6.Hrafnkelsdóttir T., Erlinge D., Jern S. Extracellular nucleotides ATP and UTP induce a marked acute release of tissue-type plasminogen activator in vivo in man. Thromb. Haemost. 2001 May;85(5):875–881. [PubMed] [Google Scholar]
- 7.Malmsjö M., Hou M., Harden T.K., Pendergast W., Pantev E., Edvinsson L., Erlinge D. Characterization of contractile P2 receptors in human coronary arteries by use of the stable pyrimidines uridine 5'-O-thiodiphosphate and uridine 5'-O-3-thiotriphosphate. J. Pharmacol. Exp. Ther. 2000 Jun;293(3):755–760. [PubMed] [Google Scholar]
- 8.Rayment S.J., Latif M.L., Ralevic V., Alexander S.P. Evidence for the expression of multiple uracil nucleotide-stimulated P2 receptors coupled to smooth muscle contraction in porcine isolated arteries. Br. J. Pharmacol. 2007 Mar;150(5):604–612. doi: 10.1038/sj.bjp.0707120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wihlborg A.K., Malmsjö M., Eyjolfsson A., Gustafsson R., Jacobson K., Erlinge D. Extracellular nucleotides induce vasodilatation in human arteries via prostaglandins, nitric oxide and endothelium-derived hyperpolarising factor. Br. J. Pharmacol. 2003 Apr;138(8):1451–1458. doi: 10.1038/sj.bjp.0705186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarowski E.R. Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 2012;8:359–373. doi: 10.1007/s11302-012-9304-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dosch M., Gerber J., Jebbawi F., Beldi G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19041222. pii: E1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gertz B.J., Haugaard E.S., Haugaard N. Effects of thyroid hormone on UTP content and uridine kinase activity of rat heart and skeletal muscle. Am. J. Physiol. 1980;238(5):E443–E449. doi: 10.1152/ajpendo.1980.238.5.E443. [DOI] [PubMed] [Google Scholar]
- 13.Olivares J., Dubus I., Barrieux A., Samuel J.L., Rappaport L., Rossi A. Pyrimidine nucleotide synthesis is preferentially supplied by exogenous cytidine in adult rat cultured cardiomyocytes. J. Mol. Cell. Cardiol. 1992;24(11):1349–1359. doi: 10.1016/0022-2828(92)93099-6. [DOI] [PubMed] [Google Scholar]
- 14.Belous A.E., Jones C.M., Wakata A., Knox C.D., Nicoud I.B., Pierce J., Chari R.S. Mitochondrial calcium transport is regulated by P2Y1- and P2Y2-like mitochondrial receptors. J. Cell. Biochem. 2006;99:1165–1174. doi: 10.1002/jcb.20985. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G. Intracellular expression of purinoceptors. Purinergic Signal. 2015;11:275–276. doi: 10.1007/s11302-015-9455-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wihlborg A.K., Balogh J., Wang L., Borna C., Dou Y., Joshi B.V., Lazarowski E., Jacobson K.A., Arne A., Erlinge D. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ. Res. 2006;98:970–976. doi: 10.1161/01.RES.0000217402.73402.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarowski E.R., Harden T.K. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br. J. Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erlinge D., Harnek J., van Heusden C., Olivecrona G., Jern S., Lazarowski E. Uridinetriphosphate (UTP) is released during cardiac ischemia. Int. J. Cardiol. 2005;100:427–433. doi: 10.1016/j.ijcard.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Yitzhaki S., Shainberg A., Cheporko Y., Vidne B.A., Sagie A., Jacobson K.A., Hochhauser E. Uridine-5'-triphosphate (UTP) reduces infarct size and improves rat heart function after myocardial infarct. Biochem. Pharmacol. 2006;72(8):949–955. doi: 10.1016/j.bcp.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen R., Shainberg A., Hochhauser E., Cheporko Y., Tobar A., Birk E., Pinhas L., Leipziger J., Don J., Porat E. UTP reduces infarct size and improves mice heart function after myocardial infarct via P2Y2 receptor. Biochem. Pharmacol. 2011;82(9):1126–1133. doi: 10.1016/j.bcp.2011.07.094. [DOI] [PubMed] [Google Scholar]
- 21.Morris J.B., Pham T.M., Kenney B., Sheppard K.E., Woodcock E.A. UTP transactivates epidermal growth factor receptors and promotes cardiomyocyte hypertrophy despite inhibiting transcription of the hypertrophic marker gene, atrial natriuretic peptide. J. Biol. Chem. 2004;279:8740–8746. doi: 10.1074/jbc.M310012200. [DOI] [PubMed] [Google Scholar]
- 22.Gergs U., Rothkirch D., Hofmann B., Treede H., Robaye B., Simm A., Müller C.E., Neumann J. Mechanism underlying the contractile activity of UTP in the mammalian heart. Eur. J. Pharmacol. 2018;830:47–58. doi: 10.1016/j.ejphar.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Zheng M., Zhang S.J., Zhu W.Z., Ziman B., Kobilka B.K., Xiao R.P. Beta 2-adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or gbeta gamma in adult mouse cardiomyocytes. J. Biol. Chem. 2000;275(51):40635–40640. doi: 10.1074/jbc.M006325200. [DOI] [PubMed] [Google Scholar]
- 24.Liao P., Wang S.Q., Wang S., Zheng M., Zheng M., Zhang S.J., Cheng H., Wang Y., Xiao R.P. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ. Res. 2002;90(2):190–196. doi: 10.1161/hh0202.104220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaikkonen L., Magga J., Ronkainen V.P., Koivisto E., Perjes Á., Chuprun J.K., Vinge L.E., Kilpiö T.1, Aro J., Ulvila J., Alakoski T., Bibb J.A., Szokodi I., Koch W.J., Ruskoaho H., Kerkelä R. p38α regulates SERCA2a function. J. Mol. Cell. Cardiol. 2014;67:86–93. doi: 10.1016/j.yjmcc.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shainberg A., Yitzhaki S., Golan O., Jacobson K.A., Hochhauser E. Involvement of UTP in protection of cardiomyocytes from hypoxic stress. Can. J. Physiol. Pharmacol. 2009;87:287–299. doi: 10.1139/Y09-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burnstock G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnstock G. Purinergic signaling in the cardiovascular system. Circ. Res. 2017;120:207–228. doi: 10.1161/CIRCRESAHA.116.309726. [DOI] [PubMed] [Google Scholar]
- 29.White P.J., Webb T.E., Boarder M.R. Characterization of a Ca2+ response to both UTP and ATP at human P2Y11 receptors: evidence for agonist-specific signaling. Mol. Pharmacol. 2003;63:1356–1363. doi: 10.1124/mol.63.6.1356. [DOI] [PubMed] [Google Scholar]
- 30.Carter R.L., Fricks I.P., Barrett M.O., Burianek L.E., Zhou Y., Ko H., Das A., Jacobson K.A., Lazarowski E.R., Harden T.K. Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol. Pharmacol. 2009;76(6):1341–1348. doi: 10.1124/mol.109.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Froldi G., Varani K., Chinellato A., Ragazzi E., Caparrotta L., Borea P.A. P2X-purinoceptors in the heart: actions of ATP and UTP. Life Sci. 1997;60:1419–1430. doi: 10.1016/s0024-3205(97)00093-3. [DOI] [PubMed] [Google Scholar]
- 32.Vassort G. Adenosine 5'-triphosphate: a P2-purinergic agonist in the myocardium. Physiol. Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson K.A., Müller C.E. Medicinal chemistry of adenosine, P2Y and P2X receptors. Neuropharmacology. 2016;104:31–49. doi: 10.1016/j.neuropharm.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz S., Navarro G., Borroto-Escuela D., Seibt B.F., Ammon Y.C., de Filippo E., Danish A., Lacher S.K., Červinková B., Rafehi M., Fuxe K., Schiedel A.C., Franco R., Müller C.E. Adenosine A2A receptor ligand recognition and signaling is blocked by A2B receptors. Oncotarget. 2018 Feb 6;9(17):13593–13611. doi: 10.18632/oncotarget.24423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersen G.Ø., Qvigstad E., Schiander I., Aass H., Osnes J.B., Skomedal T. Alpha(1)-AR-induced positive inotropic response in heart is dependent on myosin light chain phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1471–1480. doi: 10.1152/ajpheart.00232.2002. [DOI] [PubMed] [Google Scholar]
- 36.Hidaka H., Kobayashi R. Pharmacology of protein kinase inhibitors. Annu. Rev. Pharmacol. Toxicol. 1992;32:377–397. doi: 10.1146/annurev.pa.32.040192.002113. [DOI] [PubMed] [Google Scholar]
- 37.Gergs U., Böckler A., Ebelt H., Hauptmann S., Keller N., Otto V., Pönicke K., Schmitz W., Neumann J. Human 5-HT4 receptor stimulation in atria of transgenic mice. Naunyn Schmiedeberg's Arch. Pharmacol. 2013;386:357–367. doi: 10.1007/s00210-013-0831-x. [DOI] [PubMed] [Google Scholar]
- 38.Gergs U., Boknik P., Schmitz W., Simm A., Silber R.E., Neumann J. A positive inotropic effect of ATP in the human cardiac atrium. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1716–H1723. doi: 10.1152/ajpheart.00945.2007. 2008. [DOI] [PubMed] [Google Scholar]
- 39.Gergs U., Neumann J., Simm A., Silber R.E., Remmers F.O., Läer S. Phosphorylation of phospholamban and troponin I through 5-HT4-receptors in the isolated human atrium. Naunyn Schmiedeberg's Arch. Pharmacol. 2009;379:349–359. doi: 10.1007/s00210-008-0371-y. [DOI] [PubMed] [Google Scholar]
- 40.Böhm M., Meyer W., Mügge A., Schmitz W., Scholz H. Functional evidence for the existence of adenosine receptors in the human heart. Eur. J. Pharmacol. 1985;116:323–326. doi: 10.1016/0014-2999(85)90170-0. [DOI] [PubMed] [Google Scholar]
- 41.Böhm M., Pieske B., Ungerer M., Erdmann E. Characterization of A1 adenosine receptors in atrial and ventricular myocardium from diseased human hearts. Circ. Res. 1989;65:1201–1211. doi: 10.1161/01.res.65.5.1201. [DOI] [PubMed] [Google Scholar]
- 42.Froldi G., Pandolfo L., Chinellato A., Ragazzi E., Caparrotta L., Fassina G. Dual effect of ATP and UTP on rat atria: which types of receptors are involved? Naunyn Schmiedebergs Arch Pharmacol. 1994;349:381–386. doi: 10.1007/BF00170884. [DOI] [PubMed] [Google Scholar]
- 43.Burnstock G., Meghji P. The effect of adenyl compounds on the rat heart. Br. J. Pharmacol. 1983;79:211–218. doi: 10.1111/j.1476-5381.1983.tb10514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scamps F., Legssyer A., Mayoux E., Vassort G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ. Res. 1990;67:1007–1016. doi: 10.1161/01.res.67.4.1007. [DOI] [PubMed] [Google Scholar]
- 45.Dobson J.G., Jr. Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3',5'-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ. Res. 1978;43:785–792. doi: 10.1161/01.res.43.5.785. [DOI] [PubMed] [Google Scholar]
- 46.Linden J., Hollen C.E., Patel A. The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium. Correlation with cyclic adenosine monophosphate and receptor densities. Circ. Res. 1985;56:728–735. doi: 10.1161/01.res.56.5.728. [DOI] [PubMed] [Google Scholar]
- 47.Mei Q., Liang B.T. P2 purinergic receptor activation enhances cardiac contractility in isolated rat and mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H334–H341. doi: 10.1152/ajpheart.2001.281.1.H334. [DOI] [PubMed] [Google Scholar]
- 48.Mantelli L., Amerini S., Filippi S., Ledda F. Blockade of adenosine receptors unmasks a stimulatory effect of ATP on cardiac contractility. Br. J. Pharmacol. 1993;109:1268–1271. doi: 10.1111/j.1476-5381.1993.tb13759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brückner R., Fenner A., Meyer W., Nobis T.M., Schmitz W., Scholz H. Cardiac effects of adenosine and adenosine analogs in Guinea-pig atrial and ventricular preparations: evidence against a role of cyclic AMP and cyclic GMP. J. Pharmacol. Exp. Ther. 1985;234:766–774. [PubMed] [Google Scholar]
- 50.Böhm M., Brückner R., Hackbarth I., Haubitz B., Linhart R., Meyer W., Schmidt B., Schmitz W., Scholz H. Adenosine inhibition of catecholamine-induced increase in force of contraction in Guinea-pig atrial and ventricular heart preparations. Evidence against a cyclic AMP- and cyclic GMP-dependent effect. J. Pharmacol. Exp. Ther. 1984;230:483–492. [PubMed] [Google Scholar]
- 51.Podrasky E., Xu D., Liang B.T. A novel phospholipase C- and cAMP-independent positive inotropic mechanism via a P2 purinoceptor. Am. J. Physiol. Heart Circ. Physiol. 1997;273:H2380–H2387. doi: 10.1152/ajpheart.1997.273.5.H2380. [DOI] [PubMed] [Google Scholar]
- 52.von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol. Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 53.Rafehi M., Burbiel J.C., Attah I.Y., Abdelrahman A., Müller C.E. Synthesis, characterization, and in vitro evaluation of the selective P2Y2 receptor antagonist AR-C118925. Purinergic Signal. 2017;13:89–103. doi: 10.1007/s11302-016-9542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafehi M., Malik E.M., Neumann A., Abdelrahman A., Hanck T., Namasivayam V., Müller C.E., Baqi Y. Development of potent and selective antagonists for the UTP-activated P2Y4 receptor. J. Med. Chem. 2017 13;60:3020–3038. doi: 10.1021/acs.jmedchem.7b00030. [DOI] [PubMed] [Google Scholar]
- 55.Moor A.N., Fliegel L. Protein kinase-mediated regulation of the Na(+)/H(+) exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J. Biol. Chem. 1999 13;274:22985–22992. doi: 10.1074/jbc.274.33.22985. [DOI] [PubMed] [Google Scholar]
- 56.Kentish J.C. A role for the sarcolemmal Na(+)/H(+) exchanger in the slow force response to myocardial stretch. Circ. Res. 1999;85:658–660. doi: 10.1161/01.res.85.8.658. [DOI] [PubMed] [Google Scholar]
- 57.Kwon T.H., Jung H., Cho E.J., Jeong J.H., Sohn U.D. The signaling mechanism of contraction induced by ATP and UTP in feline esophageal smooth muscle cells. Mol. Cells. 2015;38:616–623. doi: 10.14348/molcells.2015.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Perjés Á., Skoumal R., Tenhunen O., Kónyi A., Simon M., Horváth I.G., Kerkelä R., Ruskoaho H., Szokodi I. Apelin increases cardiac contractility via protein kinase Cε- and extracellular signal-regulated kinase-dependent mechanisms. PLoS One. 2014;9(4) doi: 10.1371/journal.pone.0093473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weber S., Meyer-Roxlau S., El-Armouche A. Role of protein phosphatase inhibitor-1 in cardiac beta adrenergic pathway. J. Mol. Cell. Cardiol. 2016;101:116–126. doi: 10.1016/j.yjmcc.2016.09.007. [DOI] [PubMed] [Google Scholar]