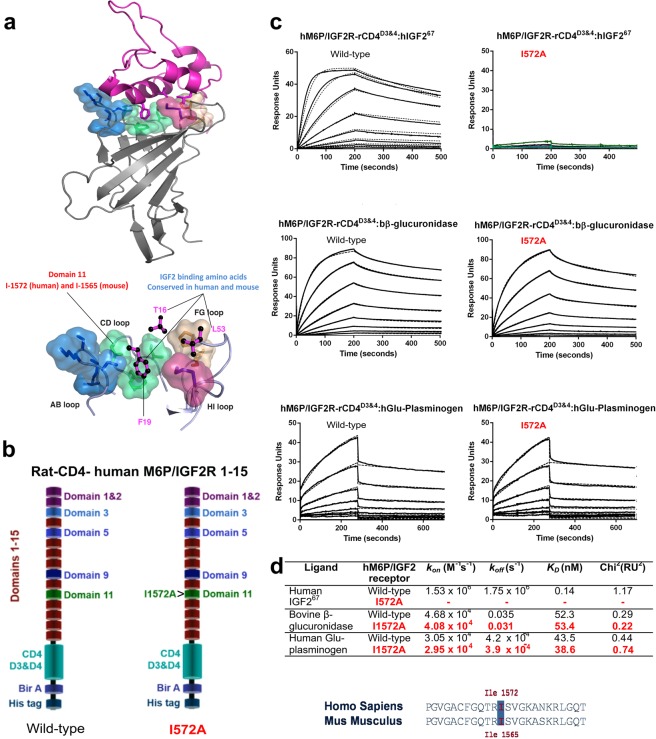
Figure 1.
Structural and molecular basis for targeting Isoleucine in the CD loop of domain 11 of M6P/IGF2R. (a) Structural configuration of human IGF2 binding to domain 11 of IGF2R (upper panel), including key interacting residues of IGF2 with CD, AB, FG and HI loops of domain 11 (lower panel). (b) Generation of a biotin acceptor site, poly-histidine (six histidines) tag and rat CD4 domains 3 + 4 fusion protein with the extra-cellular domains of M6P/IGF2R, with and without (wild-type) mutation I1572A of domain 11. Fusion proteins were expressed, purified and biotinylated as outlined in material and methods and Supplementary Fig. 1. (c) Affinity of wild-type and I1572A mutated M6P/IGF2R to human IGF2 using surface plasmon resonance. Sensorgram of the binding interactions with IGF2 (upper panel), beta-glucuronidase for M6P (middle panel) and plasminogen (lower panel), using a 1:1 binding model. Note lack of binding of IGF2 to I1572 mutant. (d) Association rates, dissociation rates and affinity constants for wild-type and I1572A interaction with ligands, including Chi2 values of their respective fits. Line up of ‘TRIS’ CD loop sequence and designation of I1572 (human) and I1565 (mouse).