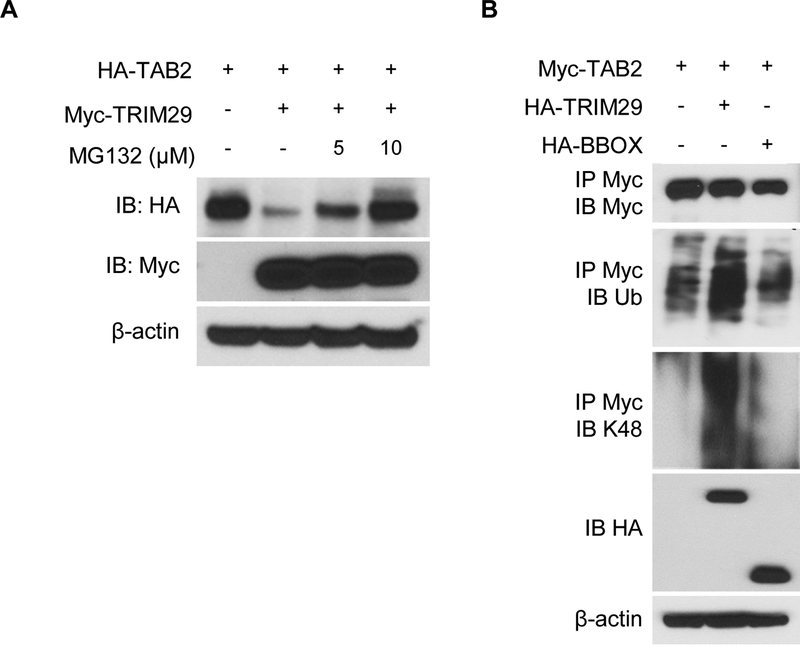
Figure 5.
TRIM29 induces ubiquitination and degradation of TAB2 via K48 linkage. (A) Immunoblotting of HA-tagged TAB2 with anti-HA (top blot) or of Myc-tagged TRIM29 with anti-Myc (second blot) in HEK293T cells transfected with empty vector or expression vector for Myc-tagged TRIM29 and treated for 4 hrs with 5 or 10 μM of MG132. β-actin was used as a loading control (bottom). (B) Immunoblotting with anti-Myc (top blot), anti-total ubiquitination (ub, 2nd blot), anti-K48-linked ubiquitination (K48, 3rd blot) of Myc-tagged TAB2 in HEK293T cells transfected with empty vector or expression vector for HA-tagged TRIM29, TRIM29-BBOX and treated for 4 hrs with 10 μM of MG132, assessed after immunoprecipitation with anti-Myc, and with anti-HA (4th blot) or anti-β-actin (bottom blot) in whole-cell lysates. Data are representative of 3 independent experiments.