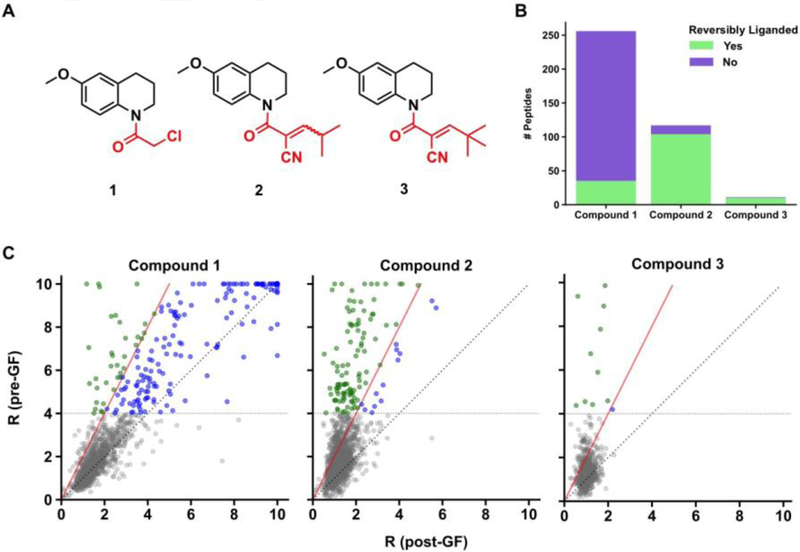
Figure 2.
Proteome-wide assessment of reversiblity of cysteine-electrophilic compound interactions by GF-isoTOP-ABPP. (A) Structures of covalent irreversible (1) and covalent reversible (2 and 3) electrophiles used in the study. (B) Bar graph showing cysteines that are liganded irreversibly (purple) or reversibly (green) by compounds 1–3. (C) Scatter plot comparisons of isoTOP-ABPP R values for cysteines before and after GF. The color coding matches that used in part B to designate cysteines that are reversibly or non-reversibly liganded by compounds 1–3. Red line denotes limit of reversibility (R ≥ 4 pre-GF and ΔR ≥ 50% post-GF). Identity line (Rpre-GF = Rpost-GF) is dotted grey. Cysteines that were not liganded (R < 4 pre-GF) are depicted in grey.