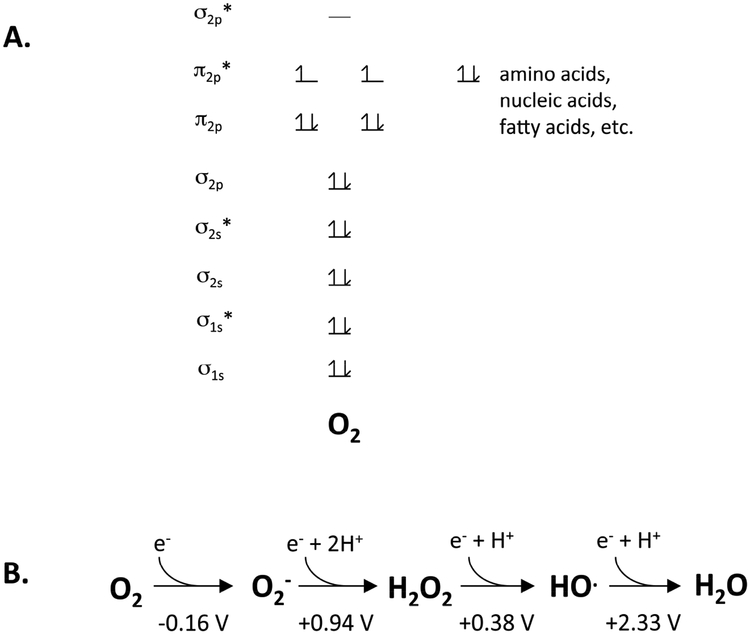
Figure 1. The chemical nature of molecular oxygen.
(A) Molecular oxygen is a di-radical. This arrangement favors rapid reaction with other radicals but permits only single-electron transfers from spin-paired biomolecules. (B) The reduction potential of oxygen indicates that it is a mediocre univalent oxidant, in contrast to superoxide, hydrogen peroxide, and the hydroxyl radical.