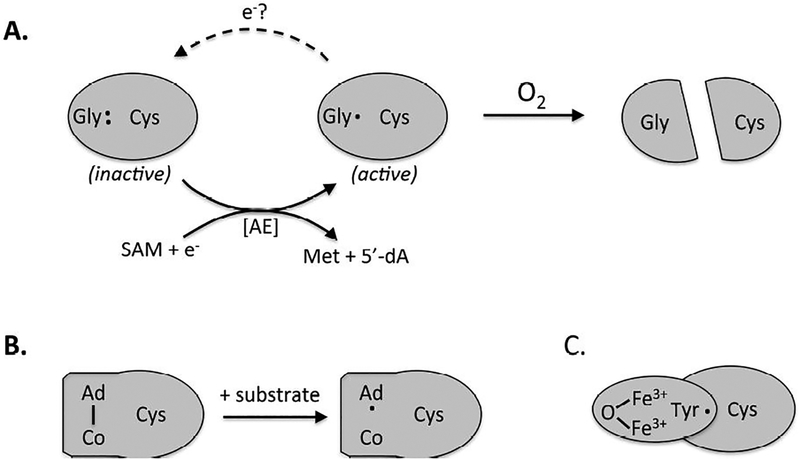
Figure 2. The evolution of oxygen-tolerant radical enzymes.
All ribonucleotide reductases maintain similar active sites, but they employ distinct mechanisms to generate the catalytic cysteinyl radical. The ancestral enzyme (A) features a resting-state glycyl radical near the protein surface, which is itself generated by a radical SAM activating enzyme (AE). Glycyl radical enzymes are rapidly inactivated by oxygen and are found only in contemporary anaerobic microbes. (B) The binuclear isozymes maintain a buried tyrosyl radical in an accessory subunit; the radical is physically insulated from the approach of oxygen. (C) In B12-dependent isozymes, an initiating adenosyl radical is generated by homolytic cleavage of the cofactor only when substrate binds, thus minimizing opportunity for reaction with O2.