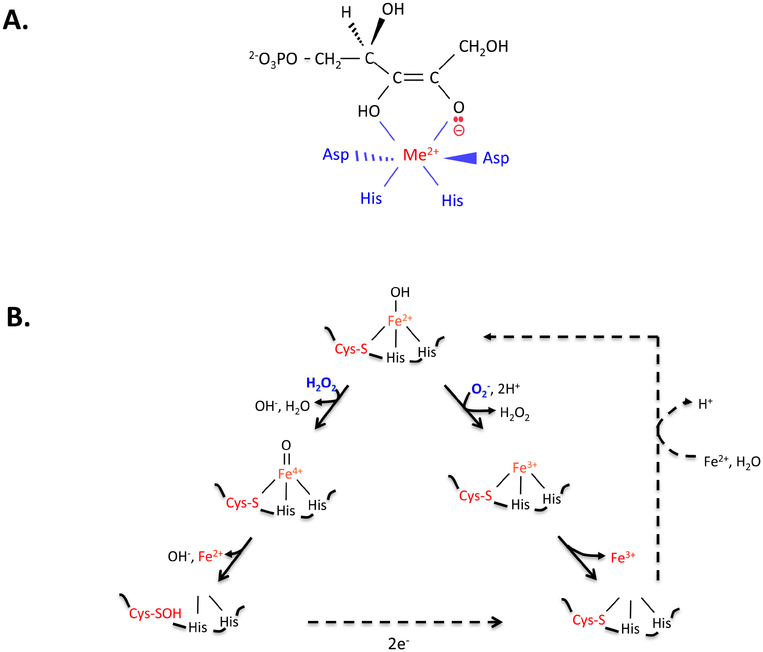
Figure 3. Non-redox iron-dependent enzymes are inactivated by hydrogen peroxide and superoxide.
(A) Mononuclear enzymes (here, ribose-5-phosphate 3-epimerase, RPE) employ an active-site divalent metal (Me2+) to stabilize anionic reaction intermediates. (B) In the substrate-free enzyme (here, peptide deformylase, PDF), the solvent exposure of the catalytic iron atom leaves it vulnerable to oxidation by H2O2 (left branch) and O2- (right). Dissociation of ferric iron inactivates the enzyme. If cysteine coordinates the metal, the ferryl radical generated by H2O2 is quickly quenched, and activity can be restored by reduction of the cysteine sulfenate residue and remetallation. Thioredoxins are likely mediators of the reduction. Some organisms employ manganese or zinc in place of iron, conferring full resistance to ROS.