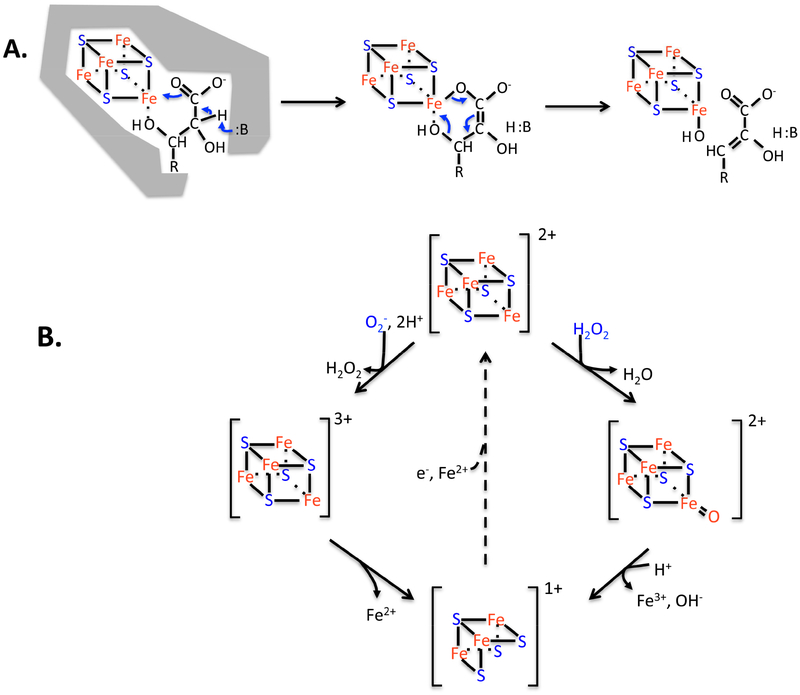
Figure 4. Dehydration by [4Fe-4S] clusters is elegant, but the enzymes are vulnerable to ROS.
(A) The unique iron atom of the solvent-exposed cluster provides an electrostatic partner that activates substrate for deprotonation, and then it serves as a Lewis acid for dehydroxylation. (B) Hydrogen peroxide and superoxide (not shown) can bind and oxidize the cluster, destabilizing it. The steady-state cluster status in vivo represents the dynamic balance between oxidation and repair. The repair process (dotted line) has been observed but has not been genetically defined. The evolution of [2Fe-2S] and cluster-free dehydratases enables some enzymes to completely resist oxidation.