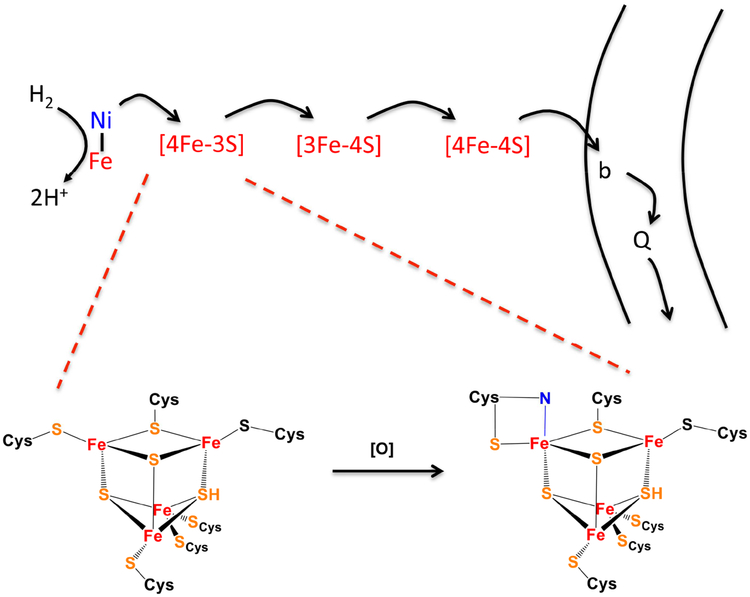
Figure 6. An unusual [4Fe-3S] cluster stabilizes membrane-bound NiFe hydrogenases against oxidation.
In soluble NiFe hydrogenases, oxygen may bind the reduced enzyme and pull electrons toward it, forming a dead-end peroxide complex that cannot be reactivated. In the membrane-bound enzymes, physiological electron flow is from hydrogen through the binuclear center and the iron-sulfur clusters to the membrane-bound b-type cytochrome and quinone pool. If oxygen binds the reduced enzyme, the back-flow of electrons to oxygen is amplified by oxidation of the proximal cluster, enabling complete oxygen reduction to water. The unusual structure of the cluster (bottom) [72] prevents disintegration of its super-oxidized form, and subsequent enzyme reduction restores full activity. In the oxidized cluster one iron atom receives bidentate coordination from the thiolate and amide nitrogen of a single cysteine residue.