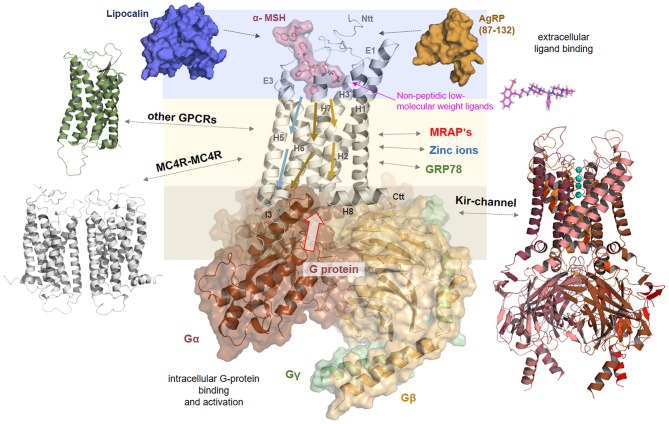
Figure 8.
Signaling regulation and putative interaction with tuning factors at the MC4R. This complex model between MC4R, α-MSH, and Gs visualizes different steps in signal transduction that are interrelated. Starting from the extracellular site, the ligand binds between the extracellular loops (E1-3) and transmembrane helices (H1-7). Ligand binding induces structural rearrangements in the transmembrane spanning part. It can be postulated that biased agonism is also associated with diversely initiated signaling pathways in the transmembrane core (colored arrows). The G-protein can fit into the receptor open crevice at the intracellular site, which is accessible in consequence to the ligand-induced receptor activation. This ternary complex finally stabilizes an active state conformation of the receptor with a defined affinity and selectivity for different intracellular effectors. In addition, the melanocortin system can be assisted by the membrane spanning dimeric (131) melanocortin receptor accessory protein (MRAP2) (132). Homo- or hetero-oligomerization of the MC4R (Figure 7) is a further regulating element in the signaling process (48). MC4R also directly or indirectly impacts the ion channel Kir 7.1 (21), is influenced by interaction with GRP78 (133), or zinc and sodium ions (52, 89). Based on a recent study (52), it can be supposed that small drug-like molecules bind into an allosteric binding site between the transmembrane helices under active support of amino acid residues at N-terminal extension.