Figure 4.
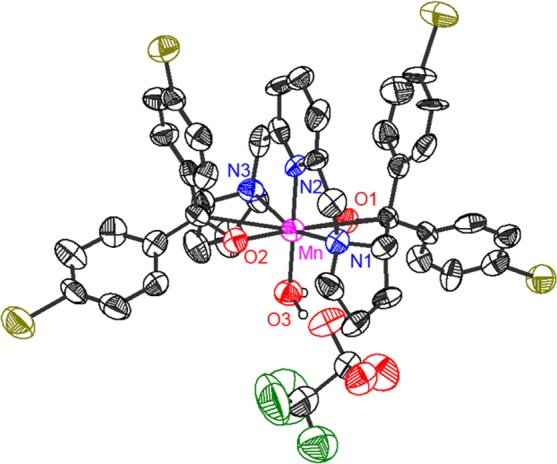
ORTEP presentation of [Mn(BDPBrP)(H2O)]OTf (6′). Hydrogen atoms are omitted for clarity. The MnIII center of 6′ possesses an octahedral coordination environment. The important bond distances and angles of 6′ are as follows: Mn–N1 = 2.248(7) Å, Mn–N2 = 2.069(7) Å, Mn–N3 = 2.255(7) Å, Mn–O1 = 1.861(5) Å, Mn–O2 = 1.881(5) Å, Mn–O3 = 2.023(5) Å; ∠N1–Mn–N2 = 77.7(3)°, ∠N2–Mn–N3 = 79.1(3)°, ∠N1–Mn–O3 = 101.5(3)°, ∠N3–Mn–O3 = 101.7(3)°, ∠O1–Mn–N2 = 90.7(3)°, ∠O2–Mn–N2 = 91.3(2)°, ∠O1–Mn–O3 = 90.5(2)°, ∠O2–Mn–O3 = 87.5(2)°.
