Abstract
Drosophila is a useful model organism for studying the molecular signatures that define specific muscle types during myogenesis. It possesses significant genetic conservation with humans for muscle disease causing genes and a lack of redundancy that simplifies functional analysis. Traditional molecular methods can be utilized to understand muscle developmental processes such as Western blots, in situ hybridizations, RT-PCR and RNAseq, to name a few. However, one challenge for these molecular methods is the ability to dissect different muscle types. In this protocol we describe some useful techniques for extracting muscles from the pupal and adult stages of development using flight and jump muscles as an example.
Keywords: Drosophila, Indirect flight muscles, Tergal depressor of trochanter, Muscular dystrophy, Myopathy, Myogenesis
1. Introduction
Muscle development and remodeling are complex processes that span from embryogenesis to the aging adult and can be dysregulated leading to disease. Degenerative diseases such as muscular dystrophies and myopathies are frequently a consequence of genetic mutations that affect the structural and contractile components of muscles [1, 2]. Striated muscle tissue in many species across the animal kingdom is typically made of different fiber types that can be identified by their morphological, biochemical, and molecular features [3–6]. The typical parameters used to characterize mammalian muscle fiber types are the expression of structural proteins (e.g., myosin heavy chain isoforms), mitochondrial content, and contractile properties (e.g., contraction speed and force) [7–9]. Muscle fibers can be loosely classified into Type I and Type II. Type I fibers have high oxidative capacity and utilize oxygen to produce continuous muscle contractions over long periods of time while type II fibers have high glycolytic activity and use anaerobic metabolism to produce short bursts of energy, but cannot maintain continuous muscle contractions for long [10]. With the advances in molecular diagnostics, fiber classification becomes even more complex and includes highly specialized fibers (e.g., periocular muscles) and transitional forms between fiber types [11, 12]. Fiber type composition of a muscle is an important parameter, as it determines overall functional performance of muscles [13] and influences systemic metabolism [14]. Mammalian muscle fibers can change their fiber-type during normal development [15], or in response to denervation and disuse [16]. Fibers of different types demonstrate different susceptibility to pathological conditions (e.g., diabetes, cancer) and aging [17–19]. Nevertheless, the exact genetic mechanisms that specify muscle diseases are currently not well understood. Given that in many cases muscle diseases can impact one fiber type to a greater extent than another, there is a need to gain a better understanding of normal human muscle development, to establish the basic knowledge required for potential treatments and therapies.
Drosophila provides a convenient experimental platform to study the heterogeneity of muscle fiber types. Most somatic muscles in adult Drosophila flies are made of multiple, but homogenous fibers. However, muscle fibers belonging to different muscles can differ substantially. From a practical standpoint, the two biggest muscle groups in Drosophila, namely indirect flight muscles (IFMs, or flight muscles) and tergal depressors of the trochanter (TDTs, or jump muscles), are a pair of muscles with highly distinct properties. Flight muscles are formed by 26 large, mitochondria-rich fibers that occupy the center of the thoracic cavity (Fig. 1). Flight muscles are highly fatigue-resistant; they power flight by slightly warping the exoskeleton of the thorax to make the wings flap, which can be done at a rate of hundreds of contractions per second. In contrast, two jump muscles are located at the opposing lateral sides of the thorax, each muscle comprising 25–30 individual fibers with low mitochondrial content. Jump muscles connect the top of the thorax with the base of the second pair of legs and, upon contraction, exclusively provide power for jumping. Flight and jump muscles demonstrate a striking difference in their morphological organization, expression of muscle genes, and alternative transcript splicing [20–22] analogous to vertebrate slow twitch (Type I) or fast twitch (Type II) fibers [12]. Thus, the conservation of muscle fiber diversity between Drosophila and vertebrates allows us to investigate the molecular mechanisms underlying muscle fiber diversity in an ideally simplified model.
Fig. 1.
Schematic overview of the muscles developing in the adult fly thorax. (a, c) Illustrated image depicting the major muscles found in the adult fly thorax: DVMs, DLMs, and TDT (b) Control adult fly showing live image of the six DLM fibers in a bisected thorax. Notice the fibers are numbered and the TDT is outlined which sits behind the DLM fibers. (d) The DLMs are removed to reveal the triangle shape fibers of the TDT which is outlined in red
Here, we present dissection protocols for the isolation of individual flight (IFMs) and jump (TDT) muscles at multiple developmental time points. These sampling approaches have been instrumental in identifying fiber-specific muscle proteins [23] and deciphering the genetic program regulating fiber-specific differentiation and pre-mRNA alternative splicing [21, 24].
2. Materials
2.1. Fly Microdissection
Binocular dissecting microscope.
Disposable plastic 100 mm petri dish lid.
22-gauge syringe needle.
Micro dissecting needle probe and needle probe holder.
1× PBS.
Rotator for microcentrifuge tubes.
1 M sucrose solution prepared in 1× PBS.
2.2. Freezing Flies and Cryosectioning
Large Petri dish lid 92 × 16 mm.
Double-sided sticky tape.
22-gauge syringe needle.
Tissue freezing medium.
Metal spatula.
Liquid Nitrogen.
Standard glass microscope slides 25 × 75 × 1 mm.
Cryotome.
2.3. Microsampling
Inverted microscope equipped with 10× and 20× objectives.
Capillary needle puller.
1.00 mm × 0.75 mm × 100 mm capillary tubes.
Injection system harness mounted on the microscope’s stage, including a needle holder, hydraulic micromanipulator, and pressurizer.
2.4. Protein Analysis
Homogenization buffer: 10 mM Potassium phosphate buffer pH 7.0, 100 mM NaCl, 2 mM MgCl2 and 2 mM EGTA, 1 mM 1,4-Dithiothreitol (DTT), 10 μM phenylmethylsulfonyl fluoride (PMSF) and a 1× protease inhibitor cocktail.
Laemmli sample buffer: 0.0313 mM Tris–HCl pH 6.8, 1% w/v SDS, 5% v/v glycerol, 0.001% w/v bromophenol blue, and 1.25% v/v 2-Mercaptoethanol.
Disposable pestles that fit into the bottom of a 1.5 mL eppendorf tube.
4–20% gradient precast polyacrylamide gel.
3. Methods
3.1. Freezing Samples for Cryosectioning and Microsampling
Obtain time 0 h pupae. 2-h larva will crawl up the side of the vial or bottle and eventually choose a site for pupariation. Once they choose their site, the larvae become shorter and extend spiracles from the head while still remaining the light white color of wandering larva. It is at this point that you can circle them and mark time zero for subsequent pupal aging.
Stage pupae appropriately to the selected hour [APF (after puparium formation)—16 h, 24 h, 48 h, 72 h, and 96 h pupae] (see Note 1).
Attach approximately 5 cm of double-sided tape to a large Petri dish lid. Using a paintbrush, gently adhere pupae to the tape in a row, positioning them side by side.
Begin removing the pupal case from the pupae by first removing the anterior/top of the pupa, then make small incisions along a line at the lateral side along the body, lift up the pupal case to reveal the thorax, and finally remove the flap from the thorax (Fig. 2a–c).
Cover pupae or anesthetized adult flies with tissue freezing medium (Fig. 2e, f).
Place a small piece of double-sided tape at the end of a metal spatula.
Place a thin layer of tissue freezing medium onto the double-sided sticky tape. Add a label to the end away from the tip of spatula.
Lay pupae/adults on their backs onto the spatula, ventral sides with legs facing up.
Arrange multiple pupae/adults closely side by side removing any large air bubbles found near or between the flies.
Take liquid nitrogen into a Styrofoam box, and tip the box so the liquid nitrogen accumulates in one corner.
Slowly lower the spatula to the liquid nitrogen surface, stop until the sample begins to whiten, and then fully submerge the sample. Wait until sample stops bubbling; discard the sample back into the liquid nitrogen with label attached. Continue freezing more samples (Fig. 2g–i).
For long storage, collect several frozen blocks in a prechilled 15 mL tube that can be stored frozen at −80 °C. Up to 15 tubes, placed horizontally, can be stored in a standard cardboard freezer box (see Notes 2 and 3).
Fig. 2.
Pupal sample preparation for cryosectioning. (a) Placed timed pupae on double-sided tape. Ensure the pupa is secure and dorsal facing upward. (b) 16-h pupa with the first step of the casing removed. (c) Continuing the removal of the case; notice the extra casing removed on either side. (d) The 16-h pupa that is finally prepared is shown where the removal is only necessary around the thorax (e) Samples are aligned onto the spatula, notice that some pupae may roll. Ensure that the pupa has not rolled with the ventral side up (f) A liberal amount of tissue freezing medium immerses the pupa in order to properly stabilize the tissue. Add a sufficient amount of tissue freezing medium to cover the samples, but not too large as a large piece may break when freezing in liquid nitrogen. (g) Prepared sample is placed in deepest corner of Styrofoam box filled with liquid nitrogen. (h) Samples are completely submerged in liquid nitrogen and removed when bubbling is minimal. (i) Samples can now be stored in a 15 mL centrifuge tube in the −80 °C freezer
3.2. Cryosectioning
Transfer a frozen block containing flies into the working chamber of a cryotome (preset to approximately −20 °C). Wait for 15 min to equilibrate the temperatures of the block and the chamber (Fig. 3a).
Adhere the block to a detachable specimen holder using freezing medium. Apply a liberal amount of freezing medium to the specimen holder and allow it to begin to turn white (Fig. 3b). For mounting, flip the frozen block to bring the side with the label and the tape on top; bring the block’s base to the specimen holder with semi-frozen freezing medium on it and let the block adhere for 10 min (Fig. 3c). Make sure that the surface with the label is in parallel with the specimen holder’s base (see Note 4).
Attach the specimen holder to the cryotome and adjust the tilt so the plane of sectioning is parallel to the surface of the block. Briefly section the block until the flies inside of the block are beginning to be exposed (Fig. 3d–f).
Section through the frozen fly samples, producing sections of >14 μm thickness.
Pick up good, even sections coming out from under the cryotome blade with a standard microscopy slide. Apply sequential sections contiguously along the slide (see Note 5).
Let cryosections dry on the slide for 15 min at room temperature (see Note 6).
Fig. 3.
Sample preparation for cryosectioning. (a) Sample and cryosection specimen holder equilibrating to the temperature of the cryostat. (b) Freezing medium added to the specimen holder is beginning to freeze. (c) Once the freezing medium has turned white, the sample is pressed into it. (d) Cryostat mount before the specimen holder is positioned. Take note of the specimen orientation ball assembly and the specimen orientation handle in black. (e) Mounted specimen holder. (f) The sample in (e) was sectioned through until the flies became visible. This is the point to start collecting sections on a slide
3.3. Microsampling Cryosections
Prepare a needle from a glass capillary tube using a needle puller.
Wear protective eyewear and trim the needle by rapidly running forceps or a metal syringe needle across the prepared glass needle tip. Some practice will be necessary to determine how far from the needle’s end the breakage has to occur. The goal here is to produce an opening of approximately 50–100 μm in diameter.
Secure the trimmed needle within the needle holder mounted on the microscope’s stage, bring it into focus, and rotate the needle to make the working edge face downward. The ideal working edge should be perpendicular to the needle’s axis (i.e., not angled) and contain no ragged surfaces (Fig. 4a, b).
Place a slide containing dried cryosections on the microscope stage, so the sections are facing upward; bring the sample into focus and move the needle tip to the center of the view, keeping it above the slide and out of focus.
Find the area of interest (e.g., cross-sectioned jump muscle, Fig. 5a–f) and lower the needle so the working edge is in a flush contact with the surface of the slide. Using micromanipulators and microscope stage controls, produce a series of successive grooves across the area of interest, converting the tissue within that area into a pile of loose material (scraps). For better precision, scraping should be performed under a 20× objective.
Switch to a smaller power 10× objective and pick up the piled tissue with a fine dissecting pin attached to a paintbrush handle (see Note 7).
Transfer amassed material at the end of the pin into an Eppendorf tube for RNA extraction. The sample can be frozen at −20 °C for long-term storage or used immediately.
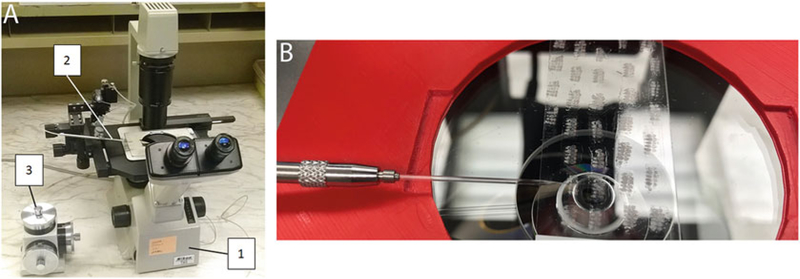
Fig. 4.
Microscope setup for microsampling. (a) Hardware setup for microsampling: an inverted microscope (1) has attached glass needle holder (2), controlled by a micromanipulator (3). (b) An example of needle placement over thick pupal sections
Fig. 5.
Identification of specific muscles for microsampling. (a) Sectioned pupa at 16 h APF, notice the white arrows indicate the area of muscle precursors of the flight muscles. (b) Sectioned pupa at 24 h APF, notice the white arrows indicate the area of muscle precursors of the flight muscles. (c, d) 48 h APF sample is sectioned, notice the black arrows indicate the myofibers of the DLMs and the arrowheads indicate the TDT. (e, f) The sample is 72 h APF, and notice the black arrows indicate the myofibers of the DLMs and the arrowheads indicate the TDT
3.4. Adult Muscle Microdissection
Obtain adult flies of desired genotypes and ages (see Note 8).
Apply a large drop of tissue freezing medium at the center of an overturned (placed upside-down) Petri dish. Anesthetize flies with CO2 (Fig. 6a), transfer and submerge the fly into tissue freezing medium (Fig. 6b–e, see Note 9).
Under a dissection microscope, cut off and discard the abdomen, the legs, the wings, and the head using the sharp edges of a 22-gauge syringe needle. Keep the liberated thorax for further work (Fig. 6e).
Using either a 22-gauge syringe needle or a microdissection probe, retrieve the thorax from tissue freezing medium, taking care to remove excess medium. Transfer the thorax into a standard microcentrifuge tube filled with 1 mL of 1× PBS. Do not load more than 10 thoraces per 1 mL of PBS; for more flies use additional tubes. Mix gently by inverting the tube for 2 min on a rotator. Let the tube stand vertically to collect thoraces at the bottom and then remove the buffer by pipette. Fill with 1 mL of fresh PBS. Repeat step 3 two more times, leave thoraces in the tube with 200 μL of PBS, place and keep on ice for the entirety of the dissection procedure (see Note 10).
Transfer a fly thorax into a 30 μL droplet of 1 M sucrose placed on the surface of an overturned Petri dish (Fig. 6f).
Split the thorax with a 22-gauge needle along the vertical midline to produce two equal hemisegments: first, insert the needle into the bigger opening on the posterior part of the thorax, where it was connected to the abdomen; next, push the needle down to make an incision through the ventral side separating the left and right legs; make a symmetrical second incision through the dorsal side to completely separate thoracic hemisegments (Fig. 6g–j).
Locate and detach an array of dorsal longitudinal muscles (DLMs)—a group of muscle fibers belonging to IFMs—inside of the dorsal part of each hemisegment. Working with a 22-gauge needle, make an incision underneath the DLM array to release a patch of cuticle with attached DLM fibers (Fig. 6k, l).
Carefully separate DLM fibers from large tracheal ducts (the latter appear as silvery/white lines running along muscle fibers) and detach the muscles from the cuticle (Fig. 6m, n).
Immediately transfer separated DLMs into a new standard microcentrifuge tube for RNA extraction or freeze at −20 °C for long storage.
After DLM removal, large vertical muscle fibers can be seen in the remaining part of the hemithorax. These are dorsoventral muscles, DVMs, that also belong to the IFMs (Fig. 1a). They are typically not collected, but they need to be removed to get access to a TDT muscle that underlies them. The jump muscle can be morphologically identified by a clearer appearance than IFM fibers and its characteristic triangular shape. The base of the triangle is attached to the dorsal cuticle while the narrow tip is connected at the base of the second leg (Figs. 1c, d and 6k, m, o, p).
Subsequently, using a 22-gauge needle, cut off the cuticle with DVM attachment sites and the bases of the first and third legs, reducing the hemithorax into a smaller, roughly triangular piece with two longer sides converging upon the base of the second leg. Locate the TDT muscle that now should be accessible in the middle of this tissue chunk and free it by cutting and removing surrounding tissues (Fig. 6l, o, p).
When the TDT muscle is free, cut away the attached cuticle and transfer the muscle into an Eppendorf tube for subsequent RNA extraction or freeze at −20 °C for longer storage (Fig. 6p). The amount of material used in this procedure is sufficient to carry out RT-PCR to identify expression of alternative transcript isoforms between muscle types (Fig. 7).
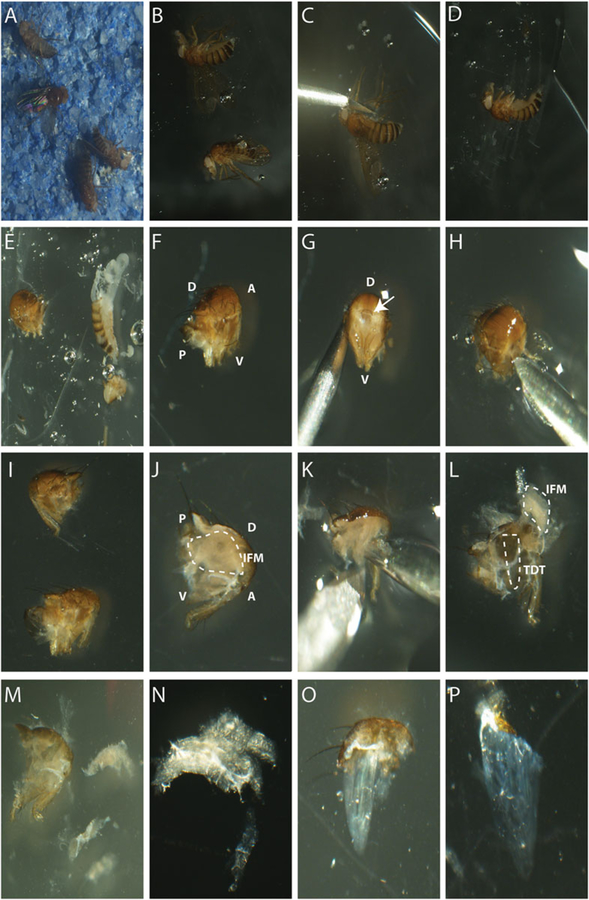
Fig. 6.
IFM and TDT dissections. (a) Anesthetized flies on CO2 pad. (b) Anesthetized flies in OCT media. (c) Removal of legs using sharp edge of the needle. (d) Complete removal of legs and wings. (e) Removal of head and abdomen in tissue freezing media using the sharp edge of a needle. (f) Thorax in 1 M Sucrose. (g) Anterior view of the thorax, arrow shows hole where head was attached. (h) Inserting the needle on the anterior side of the thorax to make vertical cut. (i) Bisected thorax. (j) Visible IFMs on bisected thorax. (k) Detaching the IFMs using tip of the needle. (l) IFMs lifted out but still attached to thorax. TDT visible underneath IFMs. (m) Extracted IFMs next to one half of the thorax. (n) Bundle of IFMs. (o) Extracted TDT with dorsal cuticle attached. (p) TDT in 1 M sucrose media. D dorsal, V ventral, A anterior, P posterior
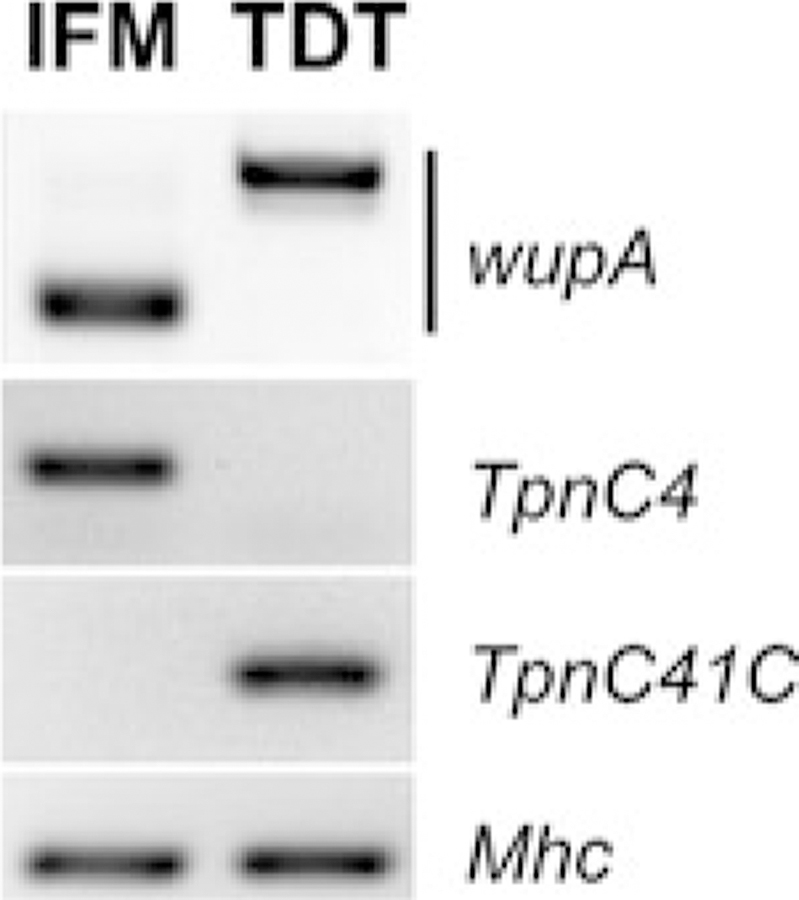
Fig. 7.
Molecular diversity of Drosophila flight and jump muscles revealed with the microsampling technique. Frozen young adults were sectioned and, under microscope control, flight (IFM) and jump (TDT) muscles were scraped and collected for RNA isolation, cDNA was made with random hexamer oligos and equalized cDNA samples were used for end-point amplification of specific gene products using sequence-specific primers. Note that this technique reveals fiber-specific preferences in alternative mRNA splicing of Troponin I transcripts (wupA), as well as mutually exclusive expression of Troponin C genes (TpnC4 and TpnC41C); amplification of pan-specific Myosin Heavy Chain (Mhc) transcripts was used as loading control
3.5. Preparation of Dissected Samples for Protein Analysis
Start with 10 or 15 dissected muscle fibers from flight or jump muscles, respectively.
Wash dissected muscles with 100 μL of homogenization buffer to remove traces of the tissue freezing medium (see Note 11).
Centrifuge muscles at 14,000 × g and carefully remove the supernatant with a narrow tip of an automatic pipette.
Add 30 μL of 1× Laemmli sample buffer and homogenize samples in a standard microcentrifuge tube using a disposable pestle.
Heat samples at 100 °C for 3 min, briefly spin down, mix by vortexing, and centrifuge at 14,000 × g for 15 min.
Dilute a sample for protein assay analysis: take 5 μL of the lysate and mix it with 20 μL of 1× Laemmli sample buffer.
Measure the concentration of protein and adjust to 2 mg/mL. Load 5 μL (i.e., 10 μg of total protein) per lane of a 4–20% gradient polyacrylamide gel.
Run protein gels in accordance with the general procedure for protein electrophoresis.
Some applications may require analysis of muscle structural proteins only. Preparations of “skinned” muscle lysates, enriched in structural proteins, can be obtained by additional treatment of dissected muscles with Triton X-100. In this case, homogenize 16 IFM or 30 TDT muscles in 100 μL of ice-cold homogenization buffer containing 0.5% Triton X-100. Centrifuge muscles at 14,000 × g, 4 °C for 5 min and carefully remove the supernatant. Wash skinned muscles three times with 100 μL of cold homogenization buffer to remove traces of Triton X-100. After centrifugation and removal of supernatant, resuspend muscles in 60 μL of 1× Laemmli sample buffer and proceed with steps 5–8 above [25].
After electrophoresis gels can be used for Coomassie staining, silver staining, or Western blotting. The amount of material (see step 1) is sufficient to visualize multiple bands in Coomassie-stained gels (Fig. 8).
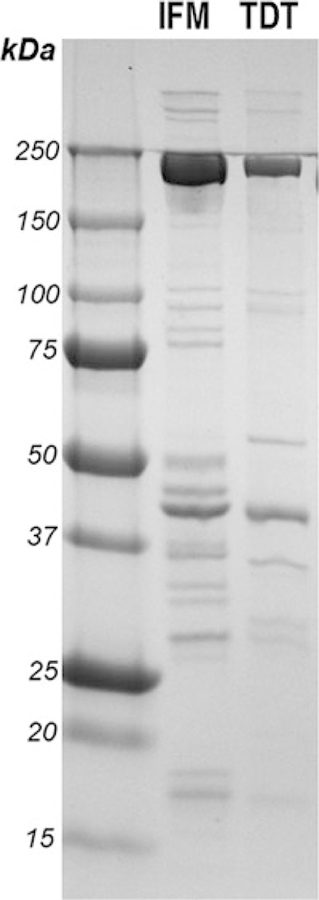
Fig. 8.
Analysis of the diversity of IFM and TDT structural proteins. IFM and TDT muscles of wild-type flies were dissected, and skinned muscle lysates were prepared according to the described protocol and procedure [25]. 10 μg of proteins from each sample were resolved in 4–20% gradient polyacrylamide gel and stained with Coomassie blue
Acknowledgments
Research at UNM is supported by grants from NIH/NIGMS: COBRE Center for Evolutionary and Theoretical Immunology (P30GM110907), the NIH (GM061738 and GM124498) and NSF (1518073) to R.M. Cripps.
4 Notes
Developmental time points need to be adjusted depending on incubation temperature.
Great care should be exercised to avoid an accidental transfer of liquid nitrogen into a tube with samples. Do not tighten tube’s cap to avoid pressure building from liquid nitrogen vapors that can potentially result in dangerous tube bursting.
Frozen blocks can be stored in a non-auto defrost −20 °C freezer, but a −80 °C freezer is preferential. During sample storage and handling, avoid warming of frozen blocks, as it will result in crystallized water formation and subsequent damage of tissues.
Applying tissue freezing medium to a cold specimen holder will lead to inadequate adhesion and may result in block separation during sectioning. Therefore, keep unused cryotome specimen holders at ambient temperature, apply freezing medium first and then place the specimen holder into the cryotome chamber for sample mounting.
We apply sections in a row following along the longer axis of the slide; when reaching slide’s edge turn the direction and produce a second row of sections. With sufficient training, a standard slide can hold six rows of sequential sections, fitting the block of five to six flies.
If necessary, air-dried slides with sections can be stored in a slide box at +4 °C, although it should be avoided for samples used for microsampling (which should always be carried out on freshly sectioned samples). Always let a box with slides sit at room temperature to equilibrate to ambient temperature before opening it. This prevents moisture from the air precipitating on sections and damaging them.
It is more practical to scrape the entire row of cryosections first, before collecting the scraped material. Lean on the microscope stage to steady the hand; use stage controls to keep the field of view in focus.
Care should be taken to ensure that control and experimental flies are of the same age, since expression of numerous genes in muscles is age-dependent. A dramatic decline in muscle structural gene expression occurs within the first 2 days post-eclosion. Dissection of flight and jump muscles from pupae is possible at the late stages of pupal development (72–96 h after puparium formation, apf), but it is difficult due to the small size of muscles and a high chance of sample contamination with histolysis products—the microsampling protocol should be used instead.
The fly’s cuticle is hydrophobic; bathing it with viscous freezing medium will prime fly surfaces for 1 M sucrose solution used in downstream dissecting procedures.
We discovered that some components of the tissue freezing medium interfere with downstream RNA purification procedures and result in lower yields of RNA. This washing step will remove freezing medium from thoraces.
It is important to remove freezing medium completely, as it may cause smudging of protein lanes during gel electrophoresis.
References
- 1.Bönnemann CG, Laing NG (2004) Myopathies resulting from mutations in sarcomeric proteins. Curr Opin Neurol 17(5):529–537 [DOI] [PubMed] [Google Scholar]
- 2.D’Amico A, Bertini E (2008) Congenital myopathies. Curr Neurol Neurosci Rep 8:73–79 [DOI] [PubMed] [Google Scholar]
- 3.Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91 (4):1447–1531 [DOI] [PubMed] [Google Scholar]
- 4.Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21 (1):51–55 [DOI] [PubMed] [Google Scholar]
- 5.Smith RS, Ovalle WK Jr (1973) Varieties of fast and slow extrafusal muscle fibres in amphibian hind limb muscles. J Anat 116(Pt 1):1–24 [PMC free article] [PubMed] [Google Scholar]
- 6.Gleeson TT, Putnam RW, Bennett AF (1980) Histochemical, enzymatic, and contractile properties of skeletal muscle fibers in the lizard Dipsosaurus dorsalis. J Exp Zool 214 (3):293–302 [DOI] [PubMed] [Google Scholar]
- 7.Thorstensson A, Grimby G, Karlsson J (1976) Force-velocity relations and fiber composition in human knee extensor muscles. J Appl Physiol 40(1):12–16 [DOI] [PubMed] [Google Scholar]
- 8.Harridge SD et al. (1996) Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432(5):913–920 [DOI] [PubMed] [Google Scholar]
- 9.Hoppeler H et al. (1973) The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch 344 (3):217–232 [DOI] [PubMed] [Google Scholar]
- 10.Herbison GJ, Jaweed MM, Ditunno JF (1982) Muscle fiber types. Arch Phys Med Rehabil 63 (5):227–230 [PubMed] [Google Scholar]
- 11.Rossi AC et al. (2010) Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles. J Physiol 588 (Pt 2):353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korfage JA, Van Eijden TM (2003) Myosin heavy-chain isoform composition of human single jaw-muscle fibers. J Dent Res 82 (6):481–485 [DOI] [PubMed] [Google Scholar]
- 13.Costill DL et al. (1976) Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40 (2):149–154 [DOI] [PubMed] [Google Scholar]
- 14.Agudelo LZ et al. (2014) Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159(1):33–45 [DOI] [PubMed] [Google Scholar]
- 15.Butler-Browne GS, Whalen RG (1984) Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol 102(2):324–334 [DOI] [PubMed] [Google Scholar]
- 16.Ciciliot S et al. (2013) Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol 45(10):2191–2199 [DOI] [PubMed] [Google Scholar]
- 17.Lexell J (1995) Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci 50:11–16 [DOI] [PubMed] [Google Scholar]
- 18.Oberbach A et al. (2006) Altered fiber distribution and fiber-specific glycolytic and oxidative enzyme activity in skeletal muscle of patients with type 2 diabetes. Diabetes Care 29 (4):895–900 [DOI] [PubMed] [Google Scholar]
- 19.Tanner CJ et al. (2002) Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282(6): E1191–E1196 [DOI] [PubMed] [Google Scholar]
- 20.Bryantsev AL et al. (2012) Differential requirements for myocyte enhancer factor-2 during adult myogenesis in Drosophila. Dev Biol 361 (2):191–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oas ST, Bryantsev AL, Cripps RM (2014) Arrest is a regulator of fiber-specific alternative splicing in the indirect flight muscles of Drosophila. J Cell Biol 206(7):895–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chechenova MB et al. (2017) Functional redundancy and non-redundancy between two troponin C isoforms in Drosophila adult muscles. Mol Biol Cell 28:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chechenova MB, Bryantsev AL, Cripps RM (2013) The Drosophila Z-disc protein Z(210) is an adult muscle isoform of Zasp52, which is required for normal myofibril organization in indirect flight muscles. J Biol Chem 288 (6):3718–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryantsev AL et al. (2012) Extradenticle and homothorax control adult muscle fiber identity in Drosophila. Dev Cell 23(3):664–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cripps RM, Sparrow JC (1992) Polymorphism in a Drosophila indirect flight muscle-specific tropomyosin isozyme does not affect flight ability. Biochem Genet 30:159. [DOI] [PubMed] [Google Scholar]