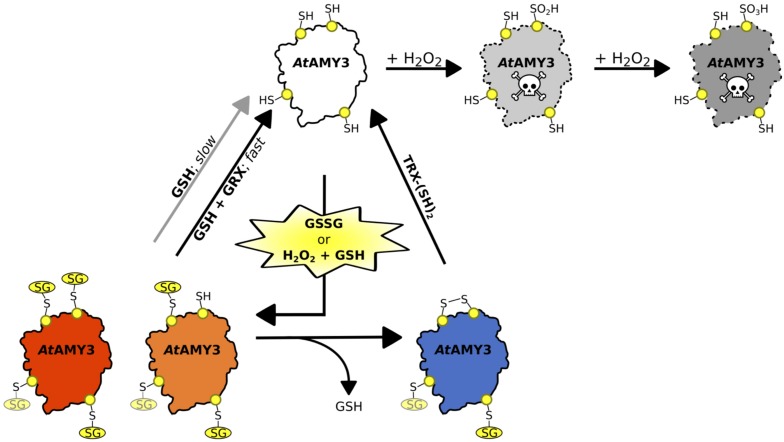
FIGURE 6.
Schematic illustration of the proposed redox-regulatory model of AtAMY3 activity. As suggested by DTNB assay, 3.5 cys residues were modified by GSSG; three of them are highlighted in yellow while the fourth is shown in light yellow. In the model, AtAMY3 (white) is fully active when reduced (–SH), but it can be irreversibly inactivated when oxidized to sulfinic (pale gray) or sulfonic (gray) acid forms by H2O2. However, if oxidation occurs in the presence of H2O2 and GSH or is mediated by GSSG, AtAMY3 is efficiently protected from overoxidation via S-glutathionylation. S-glutathionylation can lead to a partially (orange) or fully (red) modified enzyme. In case of partial S-glutathionylation (orange), an intramolecular disulfide (blue) can be formed releasing GSH. Thioredoxin (TRX) can activate AtAMY3 by reducing this disulfide (white). Similarly, fully glutathionylated AtAMY3 (red) can be efficiently activated by GSH through a slow (gray arrow) or a fast (black arrow) process depending on absence or presence of class I glutaredoxin (GRX), respectively.