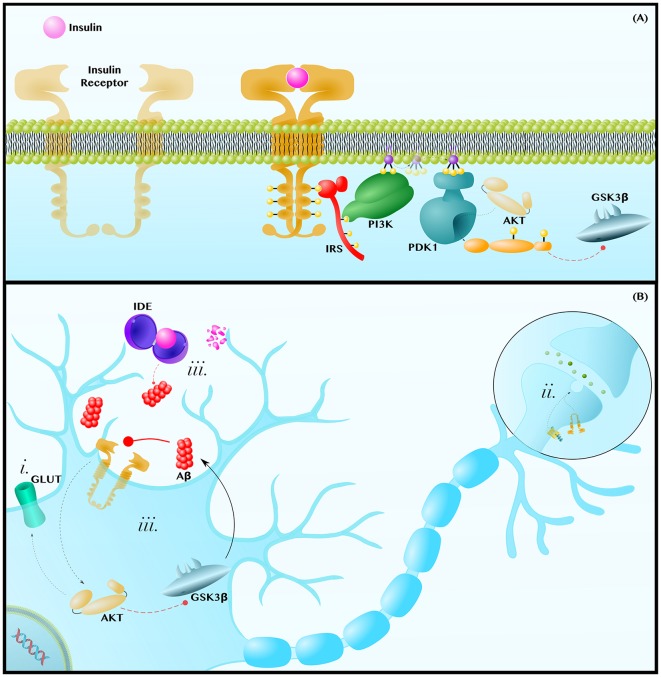
Figure 3.
Insulin resistance exacerbates the pathology of AD. (A) Insulin-AKT Pathway—Insulin binds to the Insulin Receptor (IR) tyrosine kinase, which autophosphorylates and binds the adaptor protein, Insulin Receptor Substrate (IRS). IRS recruits Phosphoinositide 3-Kinase (PI3K), which phosphorylates PIP2 into PIP3. PIP3 diffuses along the membrane to activate Phosphoinositide-Dependent Kinase 1 (PDK1), which activates AKT. AKT phosphorylates many enzymes; this includes inhibiting GSK3β. (B) Insulin Resistance Contributes to Neuropathology—Insulin resistances (i) causes a decrease in AKT-mediated translocation of GLUT transporters to the membrane. This contributes to the decreased glucose metabolic rate and mitochondrial dysfunctions observed in AD and PD brains. Insulin-AKT signaling is critical in synaptic transmission, as is Wnt-signaling. Therefore, insulin resistance (ii) may synergize with dysfunctions in Wnt-signaling to decrease synaptic transmission and synapse integrity. Lastly, insulin resistance (iii) can contribute to hyperinsulinemia and the competitive inhibition of Insulin Degrading Enzyme (IDE), which also degrades Aβ. Since Aβ inhibits insulin-AKT signaling, either insulin or Aβ can establish a positive feedback loop in which Aβ inhibits insulin signaling to decrease AKT activity, increase GSK3β activity and, thus, further increase Aβ levels. Dashed and solid lines indicate regulatory mechanisms that are, respectively, impaired and enhanced in AD.