Abstract.
Deer tick virus (DTV) is a genetic variant of Powassan virus (POWV) that circulates in North America in an enzootic cycle involving the blacklegged or “deer tick,” Ixodes scapularis, and small rodents such as the white-footed mouse. The number of reported human cases with neuroinvasive disease has increased substantially over the past few years, indicating that POWV may be of increasing public health importance. To this end, we sought to estimate POWV infection rates in questing I. scapularis collected from four health districts in Maine (York, Cumberland, Midcoast, and Central Maine). Infection rates were 1.6%, 1.7%, 0.7%, and 0%, respectively, for adults collected from April to November in 2016. Adults collected in October and November in 2017 from York and Cumberland counties had slightly higher rates of 2.3% and 3.5%, respectively. There was no difference in the number of males verses the number of females infected. All positive samples were of the DTV (lineage II) variant. Phylogenetic analysis was performed on 8 of the 15 DTV sequences obtained in 2016. Deer tick virus from the coastal regions were genetically similar and clustered with virus strains isolated from I. scapularis from New York State and Bridgeport, CT. The two inland viruses were genetically nearly identical and grouped with viruses from Massachusetts, Connecticut, and New York. These results are the first reported infection rates and sequences for POWV in questing ticks collected in Maine and will provide a reference point for future POWV studies.
INTRODUCTION
Powassan virus (POWV), Flaviviridae: Flavivirus, is a member of the tick-borne encephalitis (TBE) complex that circulates in North America and is found primarily in the upper mid-western and northeastern regions of the United States, as well as parts of Canada. It was first described from Powassan, Ontario, Canada, after a 5-year-old boy died from complications with encephalitis.1 It is the causative agent of severe central nervous infection in humans, and although still considered rare, the number of reported cases of Powassan encephalitis has risen dramatically over the past 10 years. A total of 53 cases of POWV neuroinvasive disease were reported to the CDC in 2016 and 2017, which is nearly equal to the total number of cases reported from the previous 8 years combined (2008–2015).2
Two genetic lineages of POWV circulate in nature in distinct transmission cycles—lineage I (POW) or prototype POWV occurs in an enzootic cycle mainly among Ixodes cookei and Ixodes marxi and medium-sized mammals such as skunks and groundhogs.3,4 Lineage II, or deer tick virus (DTV), is maintained in nature by the deer tick (Ixodes scapularis) and the white-footed mouse (Peromyscus leucopus) and was first described in the late 1990s.5 Although the two lineages share 94% amino acid sequence identity, they are serologically indistinguishable.6,7 Historically, both lineages have been implicated in human disease.8 Four cases of human POWV were recognized in Maine from 2000 to 2004; however, no examination of the particular lineage of the virus was performed.9 More recent human cases have been confirmed or suspected to be caused by DTV,10–12 including a fatal case of encephalitis in Maine in 2013.12 This may be due to the increasing range and distribution of I. scapularis, as well as its more frequent contact with humans.
Although human cases of POWV in Maine are well documented, the geographic distribution, rate of infection, and genetic characterization of POWV in I. scapularis ticks in Maine are not well described. In this study, we report infection rates and phylogenetic analysis of POWV in questing I. scapularis collected in Maine. We also describe implications of these findings for understanding virus transmission and disease risk in this region.
MATERIALS AND METHODS
Tick collections.
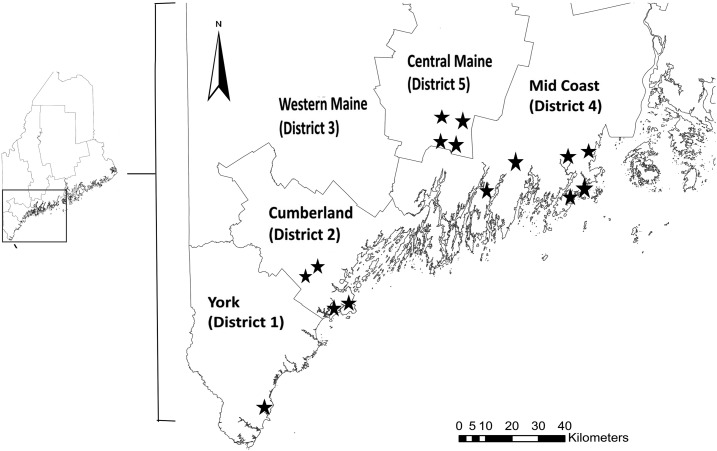
Ixodes scapularis ticks were collected from 15 field sites spanning four health districts in Maine: York, Cumberland, Midcoast, and Central Maine (Figure 1). The Maine health districts were established by the Maine Department of Health and Human services and Maine Legislature based on population size, county borders, and geographic and hospital service areas. Surveys were conducted in southern Maine in second-growth deciduous and mixed forests characterized by red oak (Quercus rubra L.), red maple (Acer rubrum L.), black cherry (Prunus serotina Ehrh.), apple (Malus spp.), red spruce (Picea rubens Sarg.), and eastern white pine (Pinus strobus L.). Where present, a dense understory layer was composed of Japanese barberry (Berberis thunbergii DC), Eurasian honeysuckle (Lonicera spp.), Asiatic bittersweet (Celastrus orbiculatus Thunb.), and high-bush blueberry (Vaccinium corymbosum L.).13,14 Successional fields, managed fields, and fresh- and saltwater wetlands were also present on survey sites. Ixodes scapularis are established across the entire study region.15
Figure 1.
Location of health districts and field collection sites (⋆) in Maine.
Field workers collected ticks by dragging a 1-m2 white corduroy flag over leaf litter and brush during each month from April through November 2016. Additional ticks were collected from the same sites in York and Cumberland health districts during October and November of the following year (2017). Ticks were transported back to the laboratory in live vials and stored at 4°C. Ticks were sorted by sex, life stage, collection site, and date collected. Only I. scapularis ticks were used in this study. Male, female, and nymphal ticks were placed in their own 2-mL plastic centrifuge tubes, whereas larvae were pooled in a group of up to 50 larvae per tube. Ticks were immediately processed for RNA isolation.
RNA isolation and polymerase chain reaction (PCR).
For ticks collected in 2016, individual ticks and pooled larvae were crushed using a sterile jumbo paperclip (5 cm size, ACCO Brands, Booneville, MS) and homogenized in 300 μL TRIzol® reagent (Invitrogen, Carlsbad, CA) by pulse vortexing with 3.2 mm diameter stainless steel beads (BioSpec Products, Bartlesville, OK). An aliquot of each individual homogenate was then combined with that of other homogenates to form pools of up to 10 adults, 20 nymphs, and 50 larvae. For example, a pool of 10 males would consist of 100-μL aliquots from 10 individuals. The pools were adjusted to a total volume of 1,000 μL TRIzol reagent, and RNA was extracted following standard protocol.16 RNA pellets were stored in 75% ethanol at −80°C and dissolved in 25 μL nuclease-free water (Growcells.com) for use in cDNA synthesis. Individual homogenates were stored in the remaining volume of TRIzol reagent at −80°C. Ticks collected in 2017 were processed in a similar manner, but with a few modifications. Ticks were ground directly in phosphate-buffered saline before pooling and then extracted using the QIAmp® Viral RNA Mini Kit (Qiagen, Germantown, MD) following the manufacturer’s protocol. A negative control of sterile, nuclease-free water (Growcells.com) was used. The positive control was a 1:100 dilution of DTV isolated from I. scapularis collected in North Branford, CT, and kindly provided by the Connecticut Agriculture Experiment Station.
Pools were screened for POWV RNA by reverse transcription polymerase chain reaction (RT-PCR) using one of two sets of primers previously designed to amplify a 291-bp region of the TBE virus complex nonstructural protein gene (NS-5) and a 689-bp region of the TBE virus envelope gene (env).5 A third set of primers, POW-bluef (5′AATCCTGTGTGACATCGGGG3ʹ) and POW-bluer (5′CCAGAGCTGCGTTGGATCTC3ʹ), which amplifies an 806-bp region of the nonstructural protein gene (NS-5), was used to confirm the presence of POWV RNA in individual ticks. Briefly, 2 μL of total RNA was used with the SuperScript™ III One-Step RT-PCR System with Platinum™ Taq DNA polymerase (Invitrogen) following standard protocol (Invitrogen). The reaction mix was cycled for 30 minutes at 50°C followed by 2 minutes at 94°C and 40 cycles of 15 seconds at 94°C, 30 seconds at 56°C, and 55 seconds at 68°C, with a final extension of 5 minutes at 68°C. cDNA was run on a 2% agarose gel and visualized using ethidium bromide staining. To confirm positivity, all positive PCRs from individual ticks were purified using the Roche High Pure PCR Product Purification Kit (Roche, Indianapolis, IN) and sequenced at the University of Maine DNA Sequencing Facility in Orono, Maine.
Genetic analysis.
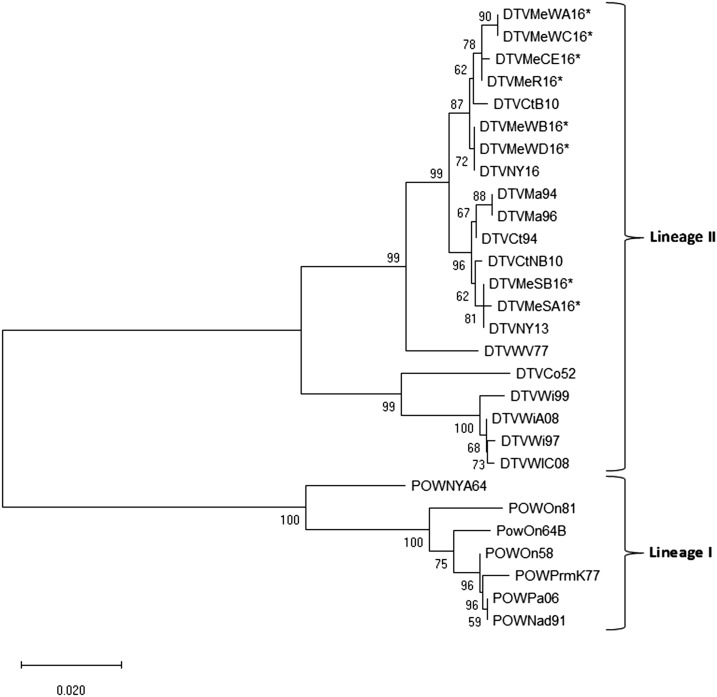
A third round of PCR was performed on eight of the positive adult ticks obtained from 2016 using the aforementioned envelope gene primers and the SuperScript III One-Step RT-PCR System with Platinum Taq high fidelity DNA polymerase (Invitrogen). Bands of appropriate size were excised from a 2% agarose gel, purified, and sequenced as described previously. Edited sequences were deposited in GenBank and assigned accession numbers (MK606358–MK606365). The eight sequences obtained from I. scapularis collected in Maine were compared with each other and with 20 other POWV sequences previously published in GenBank from samples acquired from humans, ticks, and mammals originating from Canada, Russia, and the United States (Table 1). Maine sequences were from ticks collected from Wells (DTVMeWA16-D16), Cape Elizabeth (DTVMECE16), Standish (DTVMESA16-B16), and Rockland (DTVMeR16) (Figure 2). Nucleotide sequences were cropped to a common length of 647 bp, translated into protein, and aligned by the ClustalW algorithm to preserve the integrity of the codon positions. Genetic analysis was performed with Molecular Evolutionary Genetics Analysis (Mega X) by the neighbor-joining method using the maximum composite likelihood model. One thousand boot-strap replicates were calculated to provide support for each node.
Table 1.
Previously published Powassan virus sequences used in this study
| Code* | Strain | Collection location† | Year | Host | GenBank no. |
|---|---|---|---|---|---|
| DTVNY16 | RTS84 | New York | 2016 | Ixodes scapularis | MG647783 |
| DTVNY13 | Ll-1 | New York | 2013 | I. scapularis | KJ746872 |
| DTVCtB10 | – | Bridgeport, CT | 2010 | I. scapularis | JX170780 |
| DTVCtNB10 | – | North Branford, CT | 2010 | I. scapularis | JX170772 |
| DTVWiA08 | – | Spooner, WI | 2008 | I. scapularis | HM440560 |
| DTVWiC08 | – | Spooner, WI | 2008 | I. scapularis | HM440562 |
| POWPa06 | Part/2006 | Partizansk, Russia | 2006 | Human | EU543649 |
| DTVWi99 | wicf9901 | Chippewa Falls, WI | 1999 | I. scapularis | HM440558 |
| DTVWi97 | DTV-SPO | Spooner, WI | 1997 | I. scapularis | AF310921 |
| DTVMa96 | NFS001 | Nantucket, MA | 1996 | I. scapularis | AF310918 |
| DTVCt94 | DTV-CT | Connecticut | 1994 | I. scapularis | AF310919 |
| DTVMa94 | DTV-IPS | Ipswich, MA | 1994 | I. scapularis | AF310918 |
| POWNad91 | Nad-1991 | Nadezdinsk, Russia | 1991 | Human | EU670438 |
| POWOn81 | t18-23-81 | Ontario, Canada | 1981 | Ixodes cookei | AF310909 |
| POWPrmK77 | LEIV-3070Prm | Primorsky Krai, Russia | 1977 | Ixodid tick | KT224350 |
| DTVWV77 | 12,542 | West Virginia | 1977 | Fox | AF310920 |
| PowOn64B | 1982-64 | Ontario, Canada | 1964 | Woodchuck | AF310913 |
| POWNYA64 | 64-7062 | New York | 1964 | Tick | HM440563 |
| POWOn58 | LB | Powassan, Ontario, Canada | 1958 | Human | NC003687 |
| DTVCo52 | 791A-52 | Colorado | 1952 | Dermacentor andersoni | AF310922 |
* Codes follow previously published naming convention.21
† Collection location is the United States, unless otherwise noted.
Figure 2.
Phylogenetic relationships of Powassan virus sequences analyzed by the neighbor-joining method (Mega X) of a 647-bp region of the envelope gene. The strain, state/region of origin, and year of isolation/detection are indicated for each virus. An asterisk indicates sequences generated in this study. Numbers at branch nodes represent bootstrap values.
RESULTS
Between April and November 2016, 1,653 larval, nymphal, and adult host-seeking I. scapularis ticks were collected from 15 field collection sites located in four health districts in Maine. Of the 1,206 adults, 646 were females and 560 were males (Table 2). Powassan virus RNA was detected in 15 adults: seven females (1.1%) and eight males (1.4%), by RT-PCR. There was no difference between rates of infection in females and males (Fisher’s exact test, P > 0.10). Infection rates for questing adults collected in 2016 (April–Nov) ranged from 0% (n = 117) in the most northern district tested (central Maine) to 1.6% (n = 377) and 1.7% (n = 420) in the southern districts of York and Cumberland, respectively.
Table 2.
Prevalence of Powassan virus–infected Ixodes scapularis collected in four health districts in Maine
| No. of ticks, F/M (total) | No. of deer tick virus positives, F/M | Infection rate* | |
|---|---|---|---|
| Health District (April–November 2016) | |||
| York | 181/196 (377) | 4/2 | 1.6 |
| Cumberland | 236/184 (420) | 2/5 | 1.7 |
| Midcoast | 154/138 (292) | 1/1 | 0.7 |
| Central Maine | 75/42 (117) | 0/0 | 0.0 |
| Total | 646/560 (1,206) | 7/8 | 1.2 |
| Health district (October–November 2017) | |||
| York | 180/128 (308) | 4/3 | 2.3 |
| Cumberland | 185/128 (313) | 6/5 | 3.5 |
| Total | 365/256 (621) | 10/8 | 2.9 |
* Infection rate expressed as the number of individual ticks positive/number of ticks tested.
In 2017, 621 questing adult ticks were collected in October and November from Wells (York Health District) and Cape Elizabeth (Cumberland Health District). Of these, 10 females (2.7%; n = 365) and eight males (3.1%; n = 256) were positive for POWV RNA by RT-PCR (Table 2). Infection rates for the two districts in 2017 were 2.3% (n = 308) for Wells (York Health District) and 3.5% (n = 313) for Cape Elizabeth (Cumberland Health District). There were no differences in infection rates between males and females, in years, or among health districts (Fisher’s exact tests, all P > 0.10).
Table 3 summarizes infection rates for 2016 and 2017 by life stage (spring adults [April–August], larvae, nymphs, and fall adults [September–November]). Infection rates were 1.1% in spring adults (n = 278), 0.8% in nymphs (n = 267), 0% in larvae (n = 180), and 1.3% in fall adults (n = 928) in 2016. The two positive nymphs were collected from Cape Elizabeth (Cumberland Health District) during the month of June in 2016. In 2017, fall adults had an infection rate of 2.9%. Powassan virus infection rates were not significantly different across life stages (Fisher’s exact test; P > 0.10).
Table 3.
Summary of Powassan virus detected in Ixodes scapularis collected in Maine by life stage
| Year | No. positive/No. tested (%) | |||
|---|---|---|---|---|
| Spring adults | Nymphs | Larvae | Fall adults | |
| 2016 | 3/278 (1.1%) | 2/267 (0.8%) | 0/180 (0%) | 12/928 (1.3%) |
| 2017* | ND | ND | ND | 18/621 (2.9%) |
ND = not done (ticks not collected).
*Includes only York and Cumberland health districts.
BLASTn searches in GenBank of NS-5 and env sequences obtained from all 35 POWV positive samples matched with > 98% identity to other DTV sequences deposited in GenBank. This lineage was confirmed by phylogenetic analysis of the envelope gene using eight of the 15 DTV-positive samples obtained in 2016 from I. scapularis collected from York, Cumberland, and Midcoast Health Districts along with 20 other previously published POWV env sequences in GenBank (Figure 2). Deer tick virus sequences obtained from the coastal towns of Wells, Cape Elizabeth, and Rockland, ME, were genetically similar to each other and clustered together with DTV isolates obtained from I. scapularis collected from New York State in 2016 and Bridgeport, CT, in 2010 with 87% bootstrap support. The two inland viruses originating from Standish, ME, formed a genetically homogenous subclade and grouped with viruses obtained from Massachusetts, Connecticut, and New York with 96% bootstrap support. Within the sequenced portion of the envelope gene, Standish, ME, viruses differed from Wells and Cape Elizabeth, ME, viruses by nine nucleotide substitutions and four encoded amino acid changes.
DISCUSSION
Our study reports infection prevalence of DTV by tick stage and region in Maine and demonstrates phylogenetic similarity to DTV isolates from other sites in the northeastern United States. Infection rates for questing adult I. scapularis collected from four health districts in Maine in 2016–2017 ranged from 0% to 3.5%. Our infection rates are similar to previous reports from Wisconsin, Connecticut, and New York, all which documented infection rates in the adult tick population between 0% and 5%.17–20 These studies support the idea that POWV infection rates in ticks remain relatively stable both over time and across geographic regions.17,21 For example, Brackney et al.17 reported identical infection rates in questing adult ticks collected from a northern Wisconsin focus over a 10-year span. Although we were unable to detect POWV RNA in ticks collected from our most northern health district (central Maine), this is an area where I. scapularis appears less abundant, leading to a smaller sample size. A more robust tick collection would confer more confidence in geographic and temporal variation (or lack thereof) in infection rates.
In our study, infection rates in questing spring adults collected in 2016 were nearly identical to those obtained for questing adults collected the previous fall, suggesting that overwintering has little effect on infection prevalence. In addition, we report no difference in DTV infection rates between males versus females, as is seen in other studies.17,22 Although questing nymphs appeared to have lower infection rates than adults, there was no statistically significant difference. Other studies reported lower infection rates in questing nymphs than adults, although these differences were not statistically tested.18,19,22 A higher infection rate in adults is expected due, in part, to a higher trans-stadial transmission rate for adults exposed to POWV as nymphs (54%) compared with 9.5% for nymphs exposed as larvae.23 In addition, adults have two opportunities to acquire POWV infection when feeding on vertebrate hosts as larvae and then nymphs. As any study of a low-prevalence pathogen, it was challenging to collect enough specimens to detect statistically significant differences across comparison groups. Additional studies on POWV prevalence across life stages in the natural environment are needed.
Phylogenetic analysis of a portion of the envelope gene showed, as expected, all of our samples belong to the DTV lineage. Interestingly, our samples grouped into two distinct subclades of POWV, similar to what was reported for POWV isolates obtained from I. scapularis collected in Connecticut in 2008–2011.18 Our two POWV sequences obtained from the inland site of Standish (Cumberland Health District) formed a genetically homogenous subclade from POWV sequences obtained from the coastal sites of Wells (York Health District), Cape Elizabeth (Cumberland Health District), and Rockland (Midcoast Health District), ME. One possibility for these two distinct subclades is the separate and independent introduction of POWV into these geographic locations.18 It is also possible that there are environmental factors driving this separation, which should be investigated further.
This study provides a foundation for future research on POWV in Maine and surrounding states. However, there is a great need to better characterize the molecular and ecological aspects of POWV in North America. The disparities between the presence of POWV in I. scapularis from widespread sites in the northeastern United States and upper Midwest, the rarity of human cases of encephalitis reported, and the rapidity of disease transmission to vertebrate hosts raise the possibility that more frequent but less severe human infections occur that are not diagnosed. As TBE virus pathogenicity varies across Eurasia and occurrence is often highly focal, there is a need for further studies on POWV phylogenetics, spatial distribution, and differences in strain variation and pathogenicity.
Acknowledgments:
We appreciate support provided by Michele Walsh and the Maine Department of Agriculture, Conservation, and Forestry. We would also like to thank Phil Armstrong (CT Agricultural Experiment Station) for providing us with our positive controls and feedback on the manuscript; Sam Telford (Tufts University) for his guidance through this project; and Peter W. Rand for providing helpful commentary on the final draft of this manuscript.
REFERENCES
- 1.McLean DM, Donohue WL, 1959. Powassan virus: isolation of virus from a fatal case of encephalitis. Can Med Assoc J 80: 708–711. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention , 2018. Powassan Virus Statistics and Maps. Available at: https://www.cdc.gov/powassan/statistics.html. Accessed March 25, 2019. [Google Scholar]
- 3.McLean DM, Larke RP, 1963. Powassan and Silverwater viruses: ecology of two Ontario arboviruses. Can Med Assoc J 88: 182–185. [PMC free article] [PubMed] [Google Scholar]
- 4.Main AJ, Carey AB, Downs WG, 1979. Powassan virus in Ixodes cookei and Mustelidae in New England. J Wildl Dis 15: 585–591. [DOI] [PubMed] [Google Scholar]
- 5.Telford SR, 3rd, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A, 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beasley DW, Suderman MT, Holbrook MR, Barrett AD, 2001. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res 79: 81–89. [DOI] [PubMed] [Google Scholar]
- 7.Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ, 2001. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg 65: 671–676. [DOI] [PubMed] [Google Scholar]
- 8.Ebel GD, 2010. Update on Powassan virus: emergence of a North American tick-borne Flavivirus. Annu Rev Entomol 55: 95–110. [DOI] [PubMed] [Google Scholar]
- 9.Hinten SR, et al. 2008. Increased recognition of Powassan encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis 8: 733–740. [DOI] [PubMed] [Google Scholar]
- 10.Tavakoli NP, Wang H, Dupuis M, Hull R, Ebel GD, Gilmore EJ, Faust PL, 2009. Fatal case of deer tick virus encephalitis. N Engl J Med 360: 2099–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Khoury MY, et al. 2013. Potential role of deer tick virus in Powassan encephalitis cases in Lyme disease-endemic areas of New York U.S.A. Emerg Infect Dis 19: 1926–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh CE, Muscat PL, Telford SR, 3rd, Goethert H, Pendlebury W, Elias SP, Robich R, Welch M, Lubelczyk CB, Smith RP, 2017. Fatal deer tick virus infection in Maine. Clin Infect Dis 65: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 13.Lubelczyk CB, Elias SL, Rand PW, Holman MS, LaCombe EH, Smith RP, Jr., 2004. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ Entomol 33: 900–906. [Google Scholar]
- 14.Elias SP, Lubelczyk CB, Rand PW, Lacombe EH, Holman MS, Smith RP, Jr., 2006. Deer browse resistant exotic-invasive understory: an indicator of elevated human risk of exposure to Ixodes scapularis (Acari: Ixodidae) in southern coastal Maine woodlands. J Med Entomol 43: 1142–1152. [DOI] [PubMed] [Google Scholar]
- 15.Rand PW, Lacombe EH, Dearborn R, Cahill B, Elias S, Lubelczyk CB, Beckett GA, Smith RP, Jr., 2007. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol 44: 1118–1129. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N, 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159. [DOI] [PubMed] [Google Scholar]
- 17.Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD, 2008. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 79: 971–973. [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JF, Armstrong PM, 2012. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg 87: 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aliota MT, Dupuis AP, 2nd, Wilczek MP, Peters RJ, Ostfeld RS, Kramer LD, 2014. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 14: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox KK, Thomm AM, Harrington YA, Ketter E, Patitucci JM, Carrigan DR, 2017. Powassan/deer tick virus and Borrelia burgdorferi infection in Wisconsin tick populations. Vector Borne Zoonotic Dis 17: 463–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pesko KN, Torres-Perez F, Hjelle BL, Ebel GD, 2010. Molecular epidemiology of Powassan virus in North America. J Gen Virol 91: 2698–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dupuis AP, 2nd, Peters RJ, Prusinski MA, Falco RC, Ostfeld RS, Kramer LD, 2013. Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 6: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costero A, Grayson MA, 1996. Experimental transmission of Powassan virus (Flaviviridae) by Ixodes scapularis ticks (Acari:Ixodidae). Am J Trop Med Hyg 55: 536–546. [DOI] [PubMed] [Google Scholar]