Figure 1.
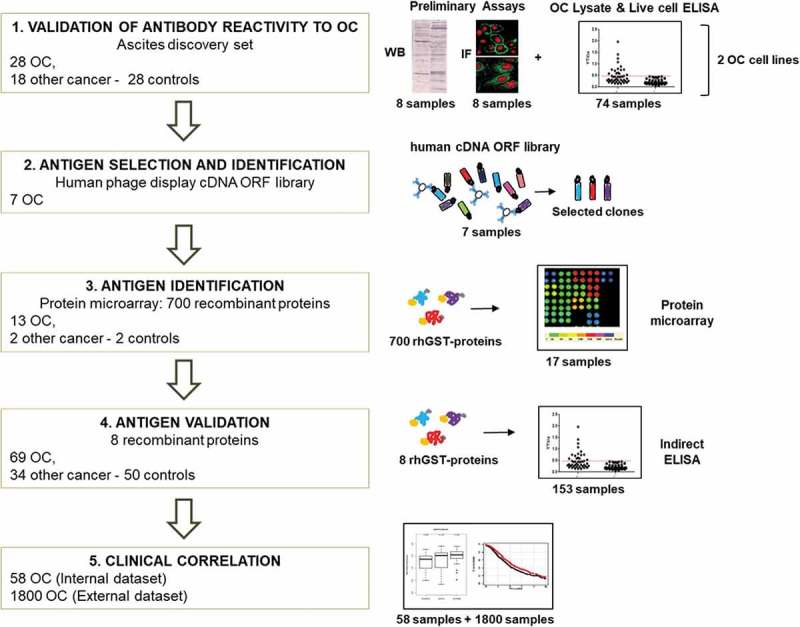
Workflow of the project.
Scheme of the project workflow and the main techniques used: 1) reactivity of antibodies from patients’ ascitic fluids against OC antigens was initially tested using a small group of samples, on two OC cell lines, by Western blot, ELISA, and immunofluorescence. 2) Antigens were identified by performing rounds of selection of an Open Reading Frame (ORF) fragments library displayed on the filamentous phage. Selection was performed with Igs from seven OC ascites. 3) Reactivity against selected putative antigens was further verified by protein microarray on 13 OC, two noncancerous, and two control samples. 4) Antigenicity validation was done by ELISA using the whole cohort of 153 ascites samples. 5) Clinical correlation of novel putative antigens to antibody titer and gene expression was performed, both on internal and external datasets.
