Fig. 2. Structural details of Twitch-2B.
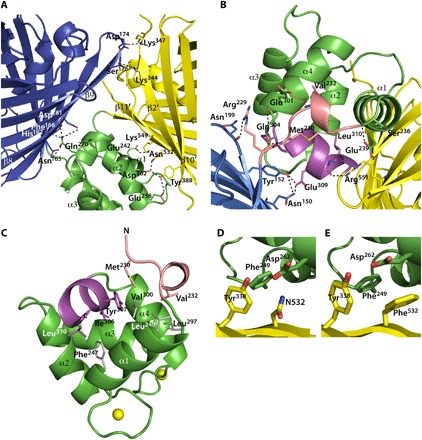
(A) Polar interactions between residues of the minimal TnC domain, mCerulean3, and cpVenuscd are depicted as dashed lines. The residues are shown as sticks. (B) Polar interactions mediated by residues (shown as sticks) from the linkers between mCerulean3 and the calcium-binding domain (in salmon), as well as interactions between cpVenuscd and the calcium-binding domain (in magenta). (C) Hydrophobic interactions between residues (shown as sticks) from the linkers between mCerulean3 and the calcium-binding domain (in salmon), as well as the linker between cpVenuscd and the calcium-binding domain (in magenta) with residues (in gray) from the core of the minimal TnC domain. (D and E) Close-up views of the region around N532 of Twitch-2B and of the N532F mutant of Twitch-2B (Twitch-6).
