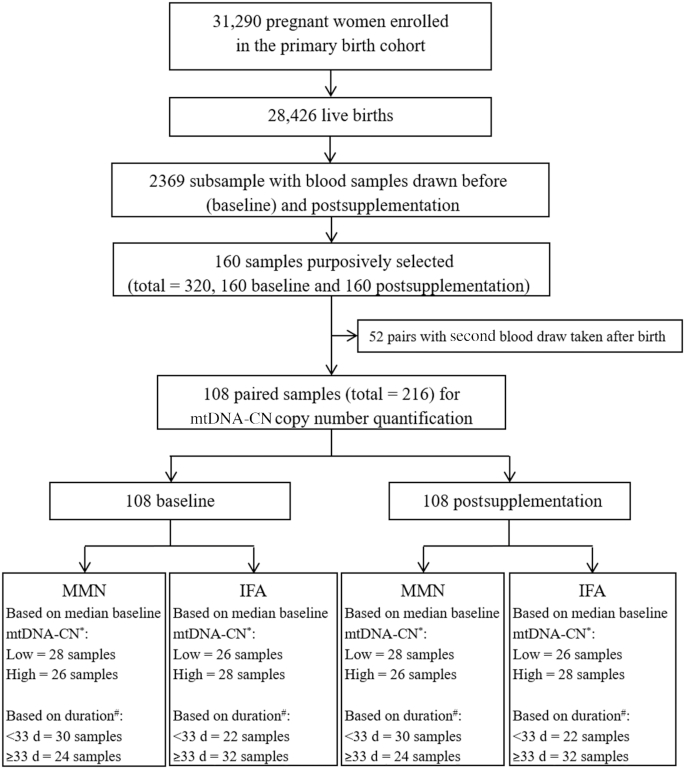
FIGURE 1.
Flowchart for mtDNA-CN assessment of 108 pregnant women enrolled in the SUMMIT study. The SUMMIT study enrolled 31,290 pregnant women in the primary birth cohort and 28,426 children were born alive. Blood samples were drawn before (baseline) and postsupplementation in a random subsample of 2369 participants. The postsupplementation blood collection was at 1 of 4 points: 1 mo after enrollment, 36 weeks of gestation, 1 wk postpartum, or 12 wk postpartum. We purposively and blindly elected 160 sets of paired baseline and postsupplementation samples. Of these samples, we selected those in which the postsupplementation blood draw was performed either 1 mo after initiation of supplementation or at ∼36 weeks of gestation (n = 108), and postpartum sets were excluded (n = 52). These 108 women were given MMN (n = 54) or IFA (n = 54) supplementation. Further, these samples were categorized as below (<33) or above (≥33) the median days of supplementation, and below (<14.1) or above (≥14.1) the median of baseline mtDNA-CN. *Low baseline < 14.1, and high baseline ≥ 14.1. #Short duration of supplementation <33 d, and long ≥33 d. IFA, iron and folic acid; MMN, multiple micronutrient; mtDNA-CN, mitochondrial DNA copy number; SUMMIT, Supplementation with Multiple Micronutrients Intervention Trial.