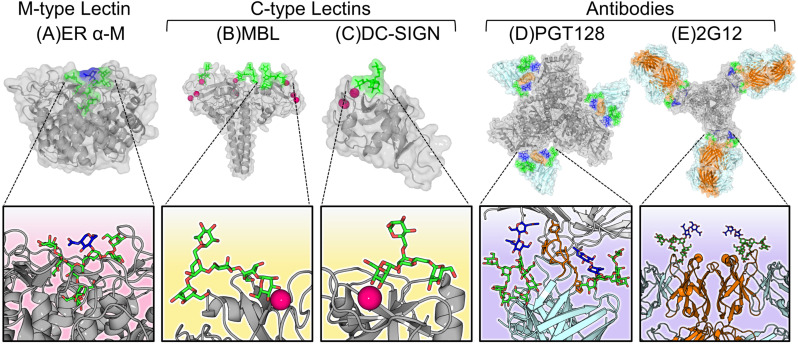
Fig. 4.
Diverse modes of oligomannose-type glycan recognition. (A) The glycan processing enzyme, ER α-mannosidase. Notice the deep binding pocket of the protein that nearly encapsulates the entire Man9GlcNAc2 glycan (PDB ID: 5KIJ) [242]. (B) The collagen-containing C-type lectin, mannose binding lectin (MBL), that recognises oligomannose-type glycans via a carbohydrate recognition domain containing a calcium ion (pink), which is responsible for mediating the primary interactions to bind the sugar (PDB ID: 1KX1) [243]. (C) The membrane bound C-type lectin receptor, DC-SIGN recognises oligomannose-type glycans via a Ca2+ ion and initiates phagocytosis of the pathogen (PDB ID: 1SL4) [216]. (D) An anti-HIV-1 broadly neutralising antibody, PGT128, that recognises conserved oligomannose-type glycans on Env using an extended complementarity-determining region (CDR) loop (orange) that penetrates the glycan shield (PDB ID: 5ACO) [244]. (E) The unique anti-oligomannose antibody, 2G12, featuring its unique “domain-exchanged” architecture where the heavy chains (orange) of the opposite Fab domain are swapped over (PDB ID: 6N2X) [245,246].