Figure 1.
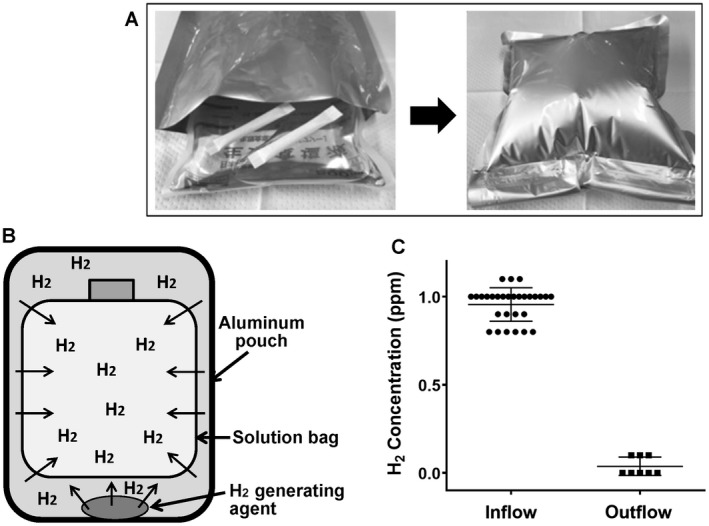
The nondestructive hydrogen dissolving method. (A and B) A small nonwoven fabric pouch containing the hydrogen‐generating agent (Ca[OH]2 mixed with aluminum powder; MiZ Co., Ltd., Kamakura, Japan) is moistened with water. Then a solution bag and 2 pieces of hydrogen‐generating agents are put together into an aluminum pouch and sealed tightly without dead space. Pure hydrogen gas is generated within the aluminum pouch, gradually permeates through the plastic bag, dissolves into the solution inside, and then reaches the plateau concentration of 1.0 ppm by 24 hours. This easy and simple method enables the preparation of H2 solution (maximally 7.0 ppm) safely, aseptically, and inexpensively (less than US $3/bag). We prepared H2 solution each time, and its concentration was measured via the methylene blue platinum colloid reagent–based titration method (dissolved hydrogen concentration measuring reagent; MiZ Co., Ltd.) as well as verified by an electrochemical gas sensor (model DHD1‐1; DKK‐TOA Corp., Tokyo, Japan). The H2 concentration was strictly maintained at 1 ppm (0.95 ± 0.02 ppm) throughout the experiments. (C) The H2 concentration in both the inflow and the outflow of the liver (just before and after the liver, respectively) was determined and is summarized here, indicating that the H2 loss before reaching the liver was minimal and that almost all H2 delivered by HyFACS was absorbed by liver tissues.
