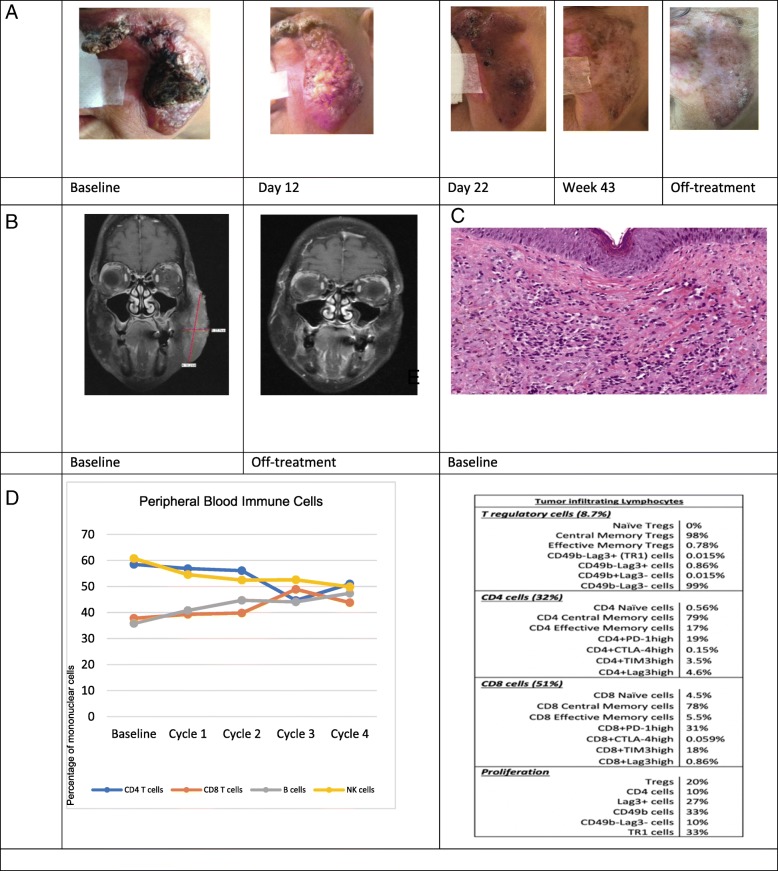
Fig. 2.
(Patient 3) a. Clinical photographs of cutaneous angiosarcoma lesion before and after treatment with AGEN1884, a monoclonal antibody to immune checkpoint CTLA-4. b. Magnetic resonance imaging before and after treatment with AGEN1884. c. Immunohistologic appearance of angiosarcoma showing malignant cells that line poorly-formed vascular lumens and infiltrate the dermis. d. Relative proportions of circulating immune cells within the peripheral blood at baseline and with subsequent treatments with AGEN1884. e. Immune phenotyping by multiparameter flow cytometry of tumor-infiltrating lymphocytes isolated from angiosarcoma tissue biopsy 12 days after the first dose of AGEN1884