Key Points
Question
Is the level of specialist care associated with premature mortality in epilepsy?
Findings
In this cohort study, when controlling for age, sex, sociodemographic factors, disease severity, and comorbidities, those receiving care from a neurologist or an epilepsy specialist had reduced risks of premature mortality compared with those unexposed to neurological care.
Meaning
The level of specialist referral is associated with an incremental reduction in the risk of premature mortality in patients who receive a diagnosis of incident epilepsy, and the greatest benefit is seen in those referred to a comprehensive epilepsy program.
Abstract
Importance
Patients with epilepsy are at an elevated risk of premature mortality. Interventions to reduce this risk are crucial.
Objective
To determine if the level of care (non-neurologist, neurologist, or comprehensive epilepsy program) is negatively associated with the risk of premature mortality.
Design, Setting, and Participants
In this retrospective open cohort study, all adult patients 18 years or older who met the administrative case definition for incident epilepsy in linked databases (Alberta Health Services administrative health data and the Comprehensive Calgary Epilepsy Programme Registry [CEP]) inclusive of the years 2002 to 2016 were followed up until death or loss to follow-up. The final analyses were performed on May 1, 2019.
Exposures
Evaluation by a non-neurologist, neurologist, or epileptologist.
Main Outcomes and Measures
The outcome was all-cause mortality. We used extended Cox models treating exposure to a neurologist or the CEP as time-varying covariates. Age, sex, socioeconomic deprivation, disease severity, and comorbid burden at index date were modeled as fixed-time coefficients.
Results
A total 23 653 incident cases were identified (annual incidence of 89 per 100 000); the mean age (SD) at index date was 50.8 (19.1) years and 12 158 (50.3%) were women. A total of 14 099 (60%) were not exposed to specialist neurological care, 9554 (40%) received care by a neurologist, and 2054 (9%) received care in the CEP. In total, 4098 deaths (71%) occurred in the nonspecialist setting, 1481 (26%) for those seen by a neurologist, and 176 (3%) for those receiving CEP care. The standardized mortality rate was 7.2% for the entire cohort, 9.4% for those receiving nonspecialist care, 5.6% for those seen by a neurologist, and 2.8% for those seen in the CEP. The hazard ratio (HR) of mortality was lower in those receiving neurologist (HR, 0.85; 95% CI, 0.77-0.93) and CEP (HR, 0.49; 95% CI, 0.38-0.62) care. In multivariable modeling, specialist care, the age at index, and disease severity were retained in the final model of the association between specialist care and mortality.
Conclusions and Relevance
Exposure to specialist care is associated with incremental reductions in the hazard of premature mortality. Those referred to a comprehensive epilepsy program received the greatest benefit.
This cohort study examines the association between the level of care received by Canadian patients with epilepsy and the risk of premature mortality.
Introduction
Epilepsy is one of the most common neurological conditions.1,2 Medications control seizures in approximately two-thirds of patients and surgery is effective in up to two-thirds of drug-resistant surgical candidates.3,4 Despite effective therapeutic options, people with epilepsy continue to have a higher standardized mortality ratio (SMR) compared with the general population. In a recent systematic review, SMRs ranged from 1.6 to 3.0 in all-age or adult populations in high-income countries across 9 studies.5
Research in kidney disease,6 cardiac disease,7 and general chronic disease8 has shown that care by specialized teams is associated with improved morbidity and lower premature mortality. Because specialized care may only be available through dedicated units or programs, patients may face barriers to access comprehensive care programs in a timely manner. Interventions associated with improved epilepsy outcomes include rational antiepileptic drug polytherapy,9 epilepsy surgery,4 dietary therapy,10,11 and mindfulness-based therapy for drug-resistant epilepsy and psychiatric comorbidities.12 In the province of Alberta, Canada, general neurology consultation is available by referral through primary care or specialist physicians. Likewise, access to epileptologist care, continuous video electroencephalogram monitoring, epilepsy-specific neuropsychological and imaging studies, and epilepsy surgery are available through referral to a comprehensive epilepsy program (CEP) via primary care or specialist physicians. Our objective was to compare mortality rates and factors associated with them in patients with epilepsy who received care at 3 levels of specialization: non-neurologist care, neurologist care, and comprehensive epilepsy care at a CEP.
Methods
Data Sources
The province of Alberta (population, 4.2 million) has a single health care system, Alberta Health Services (AHS). Two primary data repositories were leveraged to support this work. First, the AHS health data repository was used to identify health care insurance coverage, hospitalizations, physician claims, specialist care, vital statistics, and mortality. Second, patient demographic and clinical data were obtained from the clinical registry of the Calgary CEP. Alberta Health Services data were available from 2002 to 2016. Calgary CEP registry data were available from 2006 through 2016. Ethics approval for this study was obtained through the University of Calgary’s Conjoint Health Research Ethics Board. Written consent was collected for participants in the CEP. This consent separately covered the consent to (1) use program data for research and permission to (2) link administrative data resources and (3) use biologic samples for research. All participants in the CEP program data source consented to least 1 and 2.
Case Definition
A validated administrative case definition13 was used to identify epilepsy cases in patients 18 years or older. The case definition required patients to have had a minimum of 2 physician claims within a 2-year period or a single hospitalization with an epilepsy International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10 code identified in the primary, secondary, or tertiary diagnostic position. In the data sets used in this study,13 this case definition has an overall accuracy of 91.1% and sensitivity of 99.2%.
Incidence/Prevalence
A case was considered incident if the participant had a minimum of 3 years without any epilepsy claims or epilepsy admissions before the case definition satisfaction. A 2013 study14 found that a 3-year period free of epilepsy-associated claims is required to sufficiently separate incident from prevalent epilepsy cases. To assess the association of the duration of the washout period with the outcome of interest, we performed a sensitivity analysis, varying the length from 1 to 7 years.
Study Period
The study period for any participants was defined as the number of days between the index date and date of death or loss to follow-up. Death dates were captured in the provincial health care insurance registry through discharge information for participants who died in the hospital or via the provincial vital statistics database. Censorship date in persons with no mortality date was identified as the date of their final hospital discharge, physician claim, or provincial health insurance program deregistration.
Exposure
We assessed 2 types of specialized neurological care: (1) care by a neurologist and (2) care by a CEP epileptologist. Patients with at least 1 postindex physician claim containing a neurologist specialty code were considered to have received care provided by a neurologist. Participants registered with the Calgary CEP were considered to have received CEP care. Patients with no physician claims with a neurologist specialty identifier or records in the Calgary CEP registry were considered not to have received specialist neurology care.
Comorbidity
A validated epilepsy specific comorbidity index (ESCI),15,16 was used as an indicator of baseline comorbid burden. A 3-year exposure window was used to identify comorbid conditions that were proximate to the index date.
Sociodemographic Factors and Disease Severity
Two indices of sociodemographic status were included in the analysis. One index of social deprivation and a second of material deprivation were assigned using the patient’s postal code at the time of incidence.17 These indices are presented as quintile scores with increasing values representing increasing levels of deprivation. The material deprivation index (which reflects low income and education and a low employment to population ratio) and the social deprivation index (which reflects separation, living alone, widowed, or in a single-parent setting) are presented in reference to the population of Alberta. The sum of health care claims costs (in thousands of Canadian dollars) billed to the Alberta Health Care Insurance Program for each participant during the washout period and separately during the first year of incident epilepsy were used as indicators for disease severity.
Statistical Analyses
Summary statistics were calculated for each covariate for each group under comparison. Comparisons of group means were performed using a Tukey adjusted multiple comparison of means test. Comparisons of group medians were performed using a Kruskal-Wallace test of medians.
Mortality among the 3 groups under comparison (CEP, neurologist, and non-neurologist care) was compared using extended Cox models using the R statistical software package, Survival (R Foundation).18,19,20 Exposure to a neurologist or CEP was modeled as a time-varying covariate, whereas age, ESCI score at index, material deprivation, sex, severity, and social deprivation were modeled as fixed time coefficients.
Modifications of our primary association of interest by the association of age, ESCI score, material deprivation, severity, sex, and social deprivation were tested for significance using Wald tests of interaction terms. Thereafter, we assessed confounding of the association between the type of care and mortality by age, ESCI score, material deprivation, sex, severity, or social deprivation. In cases for which the inclusion of a covariate changed the estimate of the main association by more than 5%, the covariate was retained in the final model as a confounder.
To account for the baseline imbalance of age at index and severity, a propensity-matched analysis was performed.21 Propensity scores for receiving care by a neurologist or in the CEP were estimated by modeling the age at index, ESCI score at index, sex, severity as indicated by the postincidence claims total, and social deprivation. These propensity scores were used to assemble a matched cohort with balanced baseline characteristics. Tukey adjusted group means comparisons for index age and severity in this matched cohort were performed to assess the performance of the propensity matching process. An extended Cox model of mortality was used to assess the estimate of the hazard of mortality that was associated with the level of care.
A sensitivity analysis for the duration of the washout period was performed22 by running the final model on groups of patients who did not have epilepsy claims for 1 to 7 years before the index date. A second analysis was conducted to assess whether the time required to be referred and seen by the CEP could be partially responsible for the reduced mortality in participants who received CEP care and was performed using a subcohort of individuals who survived at least 2 years after the index date.
Results
Cases
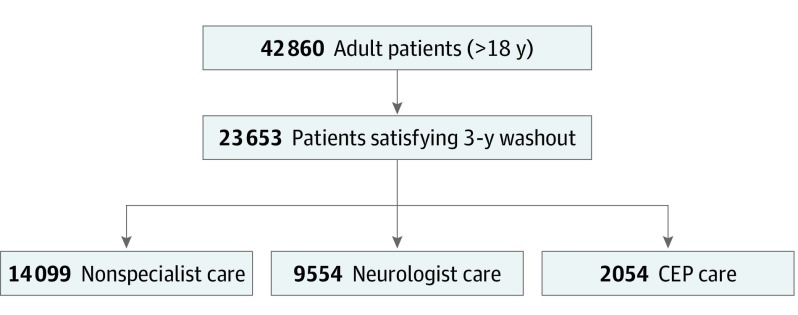
In the province of Alberta, 42 860 individuals satisfied the case definition during the study period and were 18 years or older at index date (Figure 1). Of these, 23 653 (55%) satisfied the 3-year claims-free washout period. The overall annual incidence of epilepsy was 89 cases per 100 000 and age standardized annual prevalence ranged from 1.1% to 1.4%.
Figure 1. Cohort and Exposure Flow Diagram.
CEP indicates comprehensive epilepsy program.
Exposure and Demographic Characteristics
Table 1 shows the general characteristics of patients in the 3 groups under comparison. In total, 14 099 patients (60%) did not receive neurological or CEP care, 9554 (40%) received care from a neurologist, and 2054 (9%) received care in the CEP (Figure 1). The mean (SD; interquartile range [IQR]) age at index for the entire cohort was 50.8 (19.1; 36.0-64.6) years and it was higher in patients not seen by a neurologist (53.7 [18.9; 39.8-68.0] years; P < .001) than those who received care from a neurologist (48.1 [18.9; 32.4-61.7] years) or CEP care (42.5 [16.9; 27.9-54.6] years; P < .001). The mean (SD) length of follow-up was 7.5 (2.8) years. The median ESCI score in all 3 referral groups was 0 and the IQR was 0 to 2 for those unexposed to neurologist or CEP care and 1 for the neurologist and CEP care groups (P < .001). The median social deprivation score for the full cohort was 0 to 1 and the IQR was 3 to 4 for the entire cohort; the values and IQRs for unexposed, neurologist, and CEP-exposed were 3 (3-4), 4 (3-4), and 4 (3-4), respectively. The median material deprivation score was 3 and the IQR was 2 to 4 for the entire cohort; the values and interquartile ranges for unexposed, neurologist, and cep CEP-exposed were 3 (2-4), 3 (2-4), and 3 (2-3), respectively. The median severity and IQR in thousands of Canadian dollars for washout and postincidence windows were 2.2 (0.8-5.5) and 1.0 (0.47-2.2) for the full cohort; 2.2 (0.8-5.3) and 0.9 (0.4-2.1) for the unexposed cohort; 2.4 (0.8-5.9) and 1.1 (0.6-2.3) for the neurologist exposed cohort; and 1.9 (0.7-5.1) and 0.9 (0.5-1.9) for the CEP exposed cohort, respectively.
Table 1. Demographic Characteristics of Patients With Epilepsy by Referral Group.
| Variable | Median (IQR) | |||
|---|---|---|---|---|
| All | Nonspecialist | Neurologist | CEP | |
| Female sex, No. (%) | 12 158 (50.3) | 7017 (50.0) | 4067 (50.6) | 1074 (51.6) |
| Index age, mean (SD) [IQR]a | 50.8 (19.1) [36.0-64.6] | 53.7 (18.9) [39.8-68.0] | 48.1 (18.9) [32.4-61.7] | 42.5 (16.9) [27.9-54.6] |
| Death age, mean (SD), ya | 69.1 (17.1) | 69.8 (16.8) | 67.8 (17.4) | 62.8 (17.7) |
| Index ESCI scorea | 0 (2) | 0 (2) | 0 (1) | 0 (1) |
| Follow-up, mean (SD), y | 7.5 (2.8) | 7.4 (2.9) | 7.5 (2.7) | 7.7 (2.6) |
| Material deprivation | 3 (2) | 3 (2) | 3 (2) | 3 (1) |
| Social deprivation | 4 (1) | 3 (1) | 4 (1) | 4 (1) |
| Preincident severity | 2.2 (4.7) | 2.2 (4.5) | 2.4 (5.1) | 1.9 (4.4) |
| Postincident severity | 1.0 (1.7) | 0.9 (1.7) | 1.1 (1.7) | 0.9 (1.4) |
Abbreviations: CEP, comprehensive epilepsy program; ESCI, Epilepsy Specific Comorbidity Index; IQR, interquartile range.
Significantly different across groups (P < .001).
Mortality
There were 5755 deaths; 4098 (71%) occurred in those unexposed to neurologist or CEP care, 1481 (26%) occurred in those who received care by a neurologist, and 176 (3%) occurred in those receiving CEP care. Using Statistics Canada’s most recent published mortality rate of 5.7 deaths per 1000 person-years for Alberta,23,24 the SMR was 7.2 for the entire cohort, 9.4 for those unexposed to neurological care, 5.6 for those who received care by a neurologist, and 2.8 for those who received CEP care.
Age at index, ESCI score at index, material deprivation, preincidence or postincidence severity, sex, and social deprivation did not modify the association between the type of care and mortality. Age at index and postincidence severity were the only variables that confounded the association between the type of care and mortality. After assessing modifications and confounding, care group, age at index, and postincidence severity were retained as predictors of mortality.
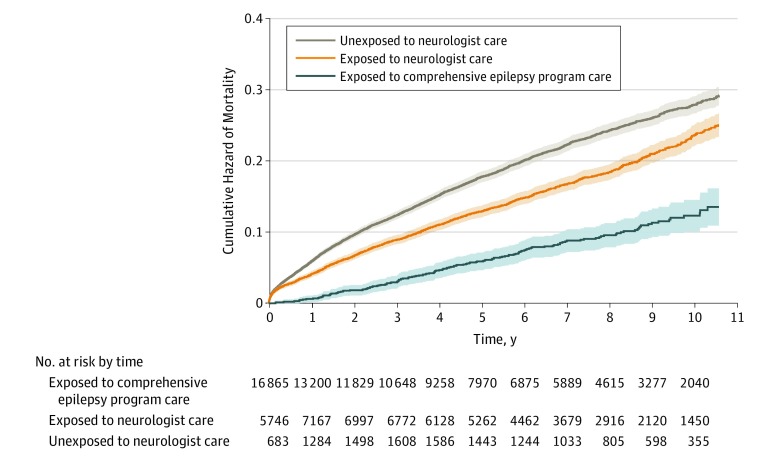
The hazard of mortality was incrementally lower for those receiving neurologist care (HR, 0.85; 95% CI, 0.77-0.93) and CEP care (HR, 0.49; 95% CI, 0.38-0.62) when compared with receiving non-neurologist care (Table 2). On the other hand, an older age at index (HR, 1.06; 95% CI, 1.06-1.06) and severity (based on claims; HR, 1.06; 95% CI, 1.05-1.07) were associated with a greater hazard of mortality (Table 2). Figure 2 shows the cumulative hazard of mortality over time adjusted by age at index and postincidence severity and stratified by the type of care. The difference between the 3 adjusted survival curves was highly significant (score [log-rank] test, 2310 on 4 df; P < .001). The final model included only those covariates that modified or confounded the association between the hazard of mortality and the level of care. In the full model, ESCI score (HR, 1.15; 95% C, 1.13-1.18), male sex (HR, 1.29; 95% CI, 1.18-1.40), and social deprivation (HR, 1.17; 95% CI, 1.12-1.23) were also significantly associated with the hazard of mortality.
Table 2. Hazard Ratio of Premature Death in Those Receiving Neurology and Epileptologist (CEP) Care as Derived From the Final Extended Cox Model of Time to Deatha.
| Term | HR (95% CI) | Standard Error | P Value |
|---|---|---|---|
| Neurologist exposure | 0.85 (0.77-0.93) | 0.05 | <.001 |
| CEP exposure | 0.49 (0.38-0.62) | 0.12 | <.001 |
| Index age | 1.06 (1.06-1.06) | 0.001 | <.001 |
| Postincident claims total | 1.06 (1.05-1.07) | 0.004 | <.001 |
Abbreviations: CEP, comprehensive epilepsy program care; HR, hazard ratio.
The HR for index age is per year. The HRs for neurologist or CEP exposure are presented as the hazard of mortality at mean age.
Figure 2. Mortality Over Time in Incident Epilepsy Cases.
An adjusted cumulative hazard curve showing the hazard of mortality over time (95% CI) in incident epilepsy cases is stratified by exposure time in terms of unexposed to neurologist care, exposed to neurologist care, and exposed to a comprehensive epilepsy care program. Exposure is a time-varying covariate. Participants may have contributed person time to multiple strata.
Propensity models for referrals to neurologists and CEP care had accuracy values of 0.55 and 0.52 and C statistics of 0.60 and 0.64, respectively. In the propensity-matched cohort, the hazard of mortality was lower in patients with epilepsy who received neurologist care compared with those who did not (HR, 0.85; 95% CI, 0.74-0.98); it was lower yet in those who received CEP care compared with those who did not (HR, 0.45; 95% CI, 0.37-0.54). Tukey adjusted group means comparisons for age or postincidence severity were insignificant in the propensity-matched cohort (.31 < P < .99).
The sensitivity analysis of the washout period showed that the association between neurologist care and mortality was not affected by the duration of the washout period. However, the association between CEP care and mortality weakened but remained significant after 5 years of washout. The sample sizes in these long washout cohorts are small, as reflected in the standard error. The SMR for the entire cohort increased from a minimum of 6.3 to a maximum of 8.0 with the increasing duration of the washout period (Table 3).
Table 3. Sensitivity of Standardized Mortality Ratios Across All Referral Groups and Hazard Ratio (SE) Between Mortality and Referral Group by Varying Length of Washout.
| Washout, y | Deaths, No. (%) | SMR | Neurologist | CEP |
|---|---|---|---|---|
| 1 | 8682 (100) | 6.3 | 0.77 (0.04) | 0.44 (0.12) |
| 2 | 7137 (82) | 6.7 | 0.80 (0.04) | 0.45 (0.12) |
| 3 | 5971 (69) | 7.0 | 0.85 (0.05) | 0.49 (0.12) |
| 4 | 4986 (57) | 7.1 | 0.91 (0.06) | 0.50 (0.14) |
| 5 | 4106 (47) | 7.3 | 0.88 (0.07) | 0.49 (0.17) |
| 6 | 3315 (38) | 7.6 | 0.86 (0.09) | 0.61 (0.20) |
| 7 | 2644 (30) | 8.0 | 0.83 (0.12) | 0.70 (0.25) |
Abbreviations: CEP, comprehensive epilepsy program care; SMR, standardized mortality ratios.
The analysis of the subcohort who were followed up for at least 2 years postincidence showed that the estimate of the association between the type of care and mortality was not meaningfully affected by removing 2142 deaths (43%) that occurred during the first 2 years of follow-up. Consistent with the main primary analysis, neurologist (HR, 0.85; 95% CI, 0.74-0.98) and CEP (HR, 0.49; 95% CI, 0.38-0.62) referral were associated with incrementally reduced hazards of premature mortality.
Discussion
These analyses demonstrate an association between types of epilepsy care and mortality. Increasing levels of specialized epilepsy care were associated with incrementally reduced hazards of premature mortality. Compared with those who did not receive neurological care, mortality rates were lower among those who received care from neurologists (HR, 0.85; 95% CI, 0.77-0.93; P < .001) and were lower still for those receiving CEP care (HR, 0.49; 95% CI, 0.38-0.63; P < .001). These results are robust to variations in case definition and the propensity of receiving different types of care. This finding supports the notion that timely referral to specialized neurological care and to a CEP appears to convey a survival benefit in incident epilepsy.
The SMR for all 3 groups was relatively high (7.0). Although comparing SMRs across populations should be done carefully,25 this figure is comparable with adult data from 2 systematic reviews.5,26 The SMR of persons receiving CEP care (2.2) is in broad agreement with the reported SMRs of 1.6 to 3.0 in population-based or community-based studies of all ages or adults only.5,26 The SMR of 5.3 for the population of individuals receiving neurologist care but not referred to a CEP is high, but it is within the range of published values. One explanation for the high SMR in these populations may be that the administrative case definition could lead to a bias toward the selection of severe cases.27 Under this circumstance, our estimates would be conservative, indicating a significant benefit from early referral to neurologist and CEP care.
There was a small upward trend in SMR as the washout period was extended in the sensitivity analysis. This could be explained by an increased mean age at onset or by a higher rate of mortality as the proportion of incident cases increased. In this setting, fewer patients were followed up for more limited time periods, and a shorter duration of enrollment in the CEP may have complicated observations about the true association between the length of the washout and our main association of interest, as indicated by higher standard errors. However, the associations remain significant despite using a 7-year washout period.
The decrease in mortality associated with specialist care is well studied for other diseases. The timing of specialist referral in kidney disease6,28 and cardiac disease29,30,31 is associated with a significant mortality benefit. Improved diagnostic performance and access to treatment options not available outside of specialist care may act as potential drivers of this benefit. The association between reduced mortality and subspecialist care could be driven by many factors. Possible causes of the observed benefit could include expediting an accurate initial diagnosis, selecting the right antiepileptic drug at the first opportunity,3 experience in using rational polytherapy,32,33 greater chances of receiving a new drug with fewer adverse effects,34 or access to epilepsy surgery.4,35
This study relied on linking clinical registry data, administrative health care data, vital statistics and provincial health care registry data sets. We successfully linked 87% of the patients in the Calgary CEP registry. Most patients in the Calgary CEP registry who could not be linked to other data sets were residents of other provinces who had no vital statistics and incomplete Alberta Health Care Insurance Plan Registry data. A few patients in the early stages of the registry had insufficient data to support linkage. Those with multiple registration/deregistration cycles in the Alberta Provincial Health Insurance Registry data set were removed from the population because of concern over access to neurologist or CEP care out of the province. Members of the Canadian Armed Forces and the Royal Canadian Mounted Police and inmates in federal penitentiaries were not included in this population.
Strengths and Limitations
The strengths of this study include a large sample size, the single data repository for all the people in the province, and accurate mortality reporting through the province’s vital statistics registry. While we have discussed potential difficulties in reliably identifying new onset epilepsy cases in administrative data, our use of an administrative case definition that was derived and validated13 in this population is also a strength of this study.
Conclusions
This study demonstrates an incremental improvement in the hazard of mortality associated with exposure to increasing levels of specialized care for adults with incident epilepsy. This finding is robust in the population under study to changes in case definition and is invariant to the level of comorbid burden and sociodemographic deprivation at incidence. The association between the level of specialist care and mortality in epilepsy merits further study. A prospective cohort study could produce valuable information to help further our understanding of the importance of referral propensity, competing risk of death, and other factors associated with mortality in epilepsy. This study allows us to conclude that in using administrative health data linked to clinical cohorts, there is a robust association between increasing levels of specialist care and improved survival in incident cases of epilepsy.
References
- 1.Fiest KM, Sauro KM, Wiebe S, et al. . Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296-303. doi: 10.1212/WNL.0000000000003509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68(5):326-337. doi: 10.1212/01.wnl.0000252807.38124.a3 [DOI] [PubMed] [Google Scholar]
- 3.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. doi: 10.1056/NEJM200002033420503 [DOI] [PubMed] [Google Scholar]
- 4.Wiebe S, Blume WT, Girvin JP, Eliasziw M; Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group . A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. doi: 10.1056/NEJM200108023450501 [DOI] [PubMed] [Google Scholar]
- 5.Thurman DJ, Logroscino G, Beghi E, et al. ; Epidemiology Commission of the International League Against Epilepsy . The burden of premature mortality of epilepsy in high-income countries: a systematic review from the Mortality Task Force of the International League Against Epilepsy. Epilepsia. 2017;58(1):17-26. doi: 10.1111/epi.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease In: Titus T, ed. Cochrane Database of Systematic Reviews. Hoboken, NJ: John Wiley & Sons, Ltd; 2014, doi: 10.1002/14651858.CD007333.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Emdin CA, Hsiao AJ, Kiran A, et al. . Referral for specialist follow-up and its association with post-discharge mortality among patients with systolic heart failure (from the National Heart Failure Audit for England and Wales). Am J Cardiol. 2017;119(3):440-444. doi: 10.1016/j.amjcard.2016.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner EH. The role of patient care teams in chronic disease management. BMJ. 2000;320(7234):569-572. doi: 10.1136/bmj.320.7234.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Louis EK. Truly “rational” polytherapy: maximizing efficacy and minimizing drug interactions, drug load, and adverse effects. Curr Neuropharmacol. 2009;7(2):96-105. doi: 10.2174/157015909788848929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kossoff EH. More fat and fewer seizures: dietary therapies for epilepsy. Lancet Neurol. 2004;3(7):415-420. doi: 10.1016/S1474-4422(04)00807-5 [DOI] [PubMed] [Google Scholar]
- 11.Martin K, Jackson CF, Levy RG, Cooper PN. Ketogenic diet and other dietary treatments for epilepsy In: Levy RG, ed. Cochrane Database of Systematic Reviews. Hoboken, NJ: John Wiley & Sons, Ltd; 2016, doi: 10.1002/14651858.CD001903.pub3. [DOI] [PubMed] [Google Scholar]
- 12.Tang V, Poon WS, Kwan P. Mindfulness-based therapy for drug-resistant epilepsy: an assessor-blinded randomized trial. Neurology. 2015;85(13):1100-1107. doi: 10.1212/WNL.0000000000001967 [DOI] [PubMed] [Google Scholar]
- 13.Reid AY, St Germaine-Smith C, Liu M, et al. . Development and validation of a case definition for epilepsy for use with administrative health data. Epilepsy Res. 2012;102(3):173-179. doi: 10.1016/j.eplepsyres.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 14.Bakaki PM, Koroukian SM, Jackson LW, Albert JM, Kaiboriboon K. Defining incident cases of epilepsy in administrative data. Epilepsy Res. 2013;106(1-2):273-279. doi: 10.1016/j.eplepsyres.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keezer MR, Bell GS, Jetté N, Sander JW. The performance of three mortality risk-adjustment comorbidity indices in a community epilepsy cohort. Epilepsia. 2015;56(5):e68-e72. doi: 10.1111/epi.12982 [DOI] [PubMed] [Google Scholar]
- 16.St Germaine-Smith C, Liu M, Quan H, Wiebe S, Jette N. Development of an epilepsy-specific risk adjustment comorbidity index. Epilepsia. 2011;52(12):2161-2167. doi: 10.1111/j.1528-1167.2011.03292.x [DOI] [PubMed] [Google Scholar]
- 17.Pampalon R, Hamel D, Gamache P, Philibert MD, Raymond G, Simpson A. An area-based material and social deprivation index for public health in Québec and Canada. Can J Public Health. 2012;103(8)(suppl 2):S17-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer; 2000. doi: 10.1007/978-1-4757-3294-8 [DOI] [Google Scholar]
- 19.R Core Team R: a language and environment for statistical computing. https://cran.r-project.org/. Accessed May 1, 2019.
- 20.Therneau TM. A package for survival analysis in S. https://www.mayo.edu/research/documents/tr53pdf/doc-10027379. Accessed May 1, 2019.
- 21.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. doi: 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sauer B, VanderWeele TJ. Sensitivity analysis In: Velentgas P, Dreyer N, Nourjah P, eds. Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality; 2013:177-184. [PubMed] [Google Scholar]
- 23.Statistics Canada Report on the demographic situation in Canada. https://www150.statcan.gc.ca/n1/pub/91-209-x/91-209-x2013001-eng.htm. Accessed May 1, 2019.
- 24.Statistics Canada Number of deaths and crude death rate per 1,000 population, Canada, provinces and territories, 1981 to 2011; number of deaths and crude death rate per 1000 population (2015). http://www.statcan.gc.ca/pub/91-209-x/2013001/article/11867/tbl/tbl1-eng.htm. Accessed July 25, 2017.
- 25.Shackleton DP, Westendorp RGJ, Kasteleijn-Nolst Trenité DGA, de Craen AJM, Vandenbroucke JP. Survival of patients with epilepsy: an estimate of the mortality risk. Epilepsia. 2002;43(4):445-450. doi: 10.1046/j.1528-1157.2002.10301.x [DOI] [PubMed] [Google Scholar]
- 26.Forsgren L, Hauser WA, Olafsson E, Sander JW, Sillanpää M, Tomson T. Mortality of epilepsy in developed countries: a review. Epilepsia. 2005;46(suppl 11):18-27. doi: 10.1111/j.1528-1167.2005.00403.x [DOI] [PubMed] [Google Scholar]
- 27.Tomson T. Mortality in epilepsy. J Neurol. 2000;247(1):15-21. doi: 10.1007/s004150050004 [DOI] [PubMed] [Google Scholar]
- 28.Smart NA, Titus TT. Outcomes of early versus late nephrology referral in chronic kidney disease: a systematic review. Am J Med. 2011;124(11):1073-80.e2. doi: 10.1016/j.amjmed.2011.04.026 [DOI] [PubMed] [Google Scholar]
- 29.Natarajan MK, Mehta SR, Holder DH, et al. . The risks of waiting for cardiac catheterization: a prospective study. CMAJ. 2002;167(11):1233-1240. [PMC free article] [PubMed] [Google Scholar]
- 30.Alter DA, Newman AM, Cohen EA, Sykora K, Tu JV. The evaluation of a formalized queue management system for coronary angiography waiting lists. Can J Cardiol. 2005;21(13):1203-1209. [PubMed] [Google Scholar]
- 31.Knudtson ML, Beanlands R, Brophy JM, Higginson L, Munt B, Rottger J; Canadian Cardiovascular Society Access to Care Working Group . Treating the right patient at the right time: access to specialist consultation and non-invasive testing. Can J Cardiol. 2006;22(10):819-824. doi: 10.1016/S0828-282X(06)70299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodie MJ, Mumford JP. Double-blind substitution of vigabatrin and valproate in carbamazepine-resistant partial epilepsy. 012 Study group. Epilepsy Res. 1999;34(2-3):199-205. doi: 10.1016/S0920-1211(98)00110-7 [DOI] [PubMed] [Google Scholar]
- 33.Brodie MJ, Yuen AW. Lamotrigine substitution study: evidence for synergism with sodium valproate? 105 Study Group. Epilepsy Res. 1997;26(3):423-432. doi: 10.1016/S0920-1211(96)01007-8 [DOI] [PubMed] [Google Scholar]
- 34.Faught E, Helmers SL, Begley CE, et al. . Newer antiepileptic drug use and other factors decreasing hospital encounters. Epilepsy Behav. 2015;45:169-175. doi: 10.1016/j.yebeh.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 35.Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia. 2003;44(4):575-581. doi: 10.1046/j.1528-1157.2003.03302.x [DOI] [PubMed] [Google Scholar]