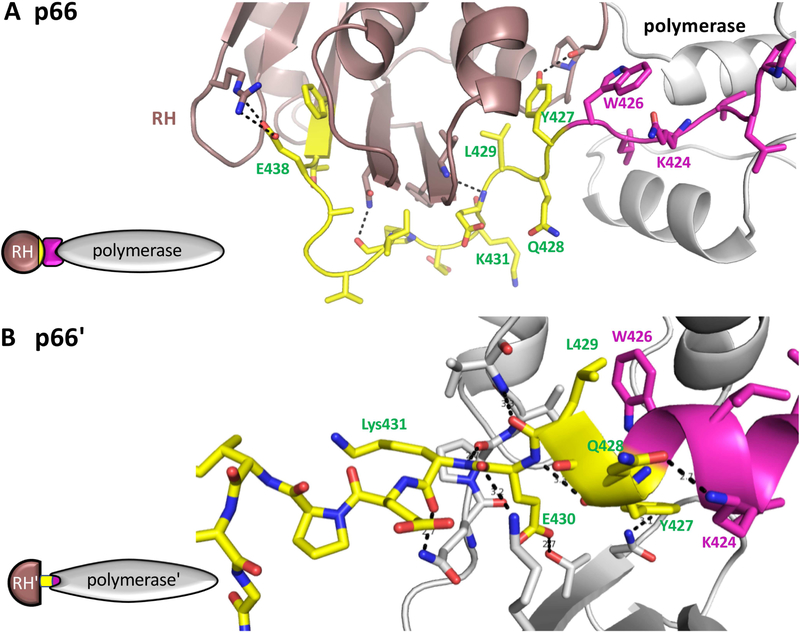
Figure 4. Subunit-specific "tug-of-war" for N-terminal RH domain residues.
In the p66 subunit (A), the C-terminal segment of the connection domain (magenta) adopts an extended conformation that links the polymerase (gray) and RH (brown) domains. The N-terminal segment of the RH domain from Tyr427-Tyr440 is colored yellow. In the p66' subunit (B), The polymerase domain C-terminal residues form a helix (magenta) that extends to include several residues derived from the RH' domain (yellow) when this domain is transiently unfolded. In the structure shown (pdb: 1RTJ, (Esnouf et al., 1995)), 8 hydrogen bonds are shown between residues derived from the RH' domain and the polymerase' domain, and additional interactions include the hydrophobic interface between Leu429 and Trp426. In both panels, the polymerase domain is gray, except for the C-terminal residues (magenta); the N-terminal RH domain residues Tyir427-Phe440 are yellow, and the remaining RH domain residues are brown. In panel B, the sidechain conformations of Q242 and Q428 were adjusted relative to structure 1RTJ to optimize the H-bond interactions. The schematics on the left illustrate the inclusion of RH' domain N-terminal residues (yellow) into the polymerase' domain.