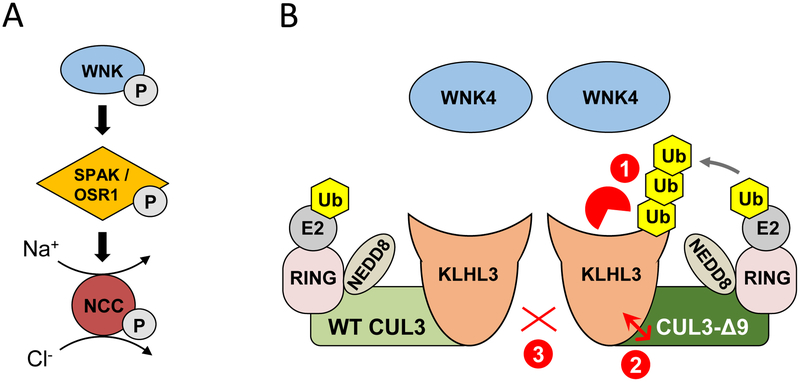
Figure 2. Normal and abnormal regulation of the renal Na+-Cl− cotransporter (NCC).
A) NCC is activated via phosphorylation by SPAK or the related kinase OSR1, which are in turn phosphorylated and activated by WNK kinases. B) Dimers of wild type (WT) CUL3-containing CRLs suppress NCC activation by mediating WNK4 degradation (not shown). Cul3 mutations cause the human disease Familial Hyperkalemic Hypertension (FHHt) due skipping of exon 9 during RNA splicing, leading to expression of CUL3-Δ9. Several mechanisms by which CUL3-Δ9 cause FHHt have been proposed. 1) CUL3-Δ9 inappropriately tags the WNK substrate adaptor KLHL3 for degradation; 2) CUL3-Δ9 has increased affinity for KLHL3, and sequesters it in non-functional CRLs; 3) WT and CUL3-Δ9 interact weakly. In each case, the loss of functional CRLs would result in accumulation of WNK4, and hyperactivation of NCC.