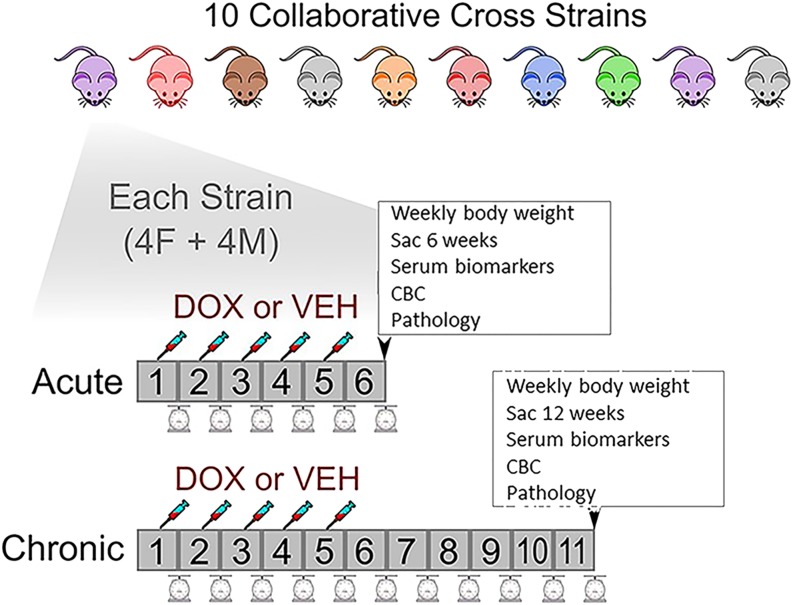
Figure 1.
Study design. 10 CC strains were selected. 32 mice per line equally split by sex and treatment regimen (acute and recovery doxorubicin treatment and control groups) were used. A total of 25 mg/kg of DOX (5 tail injections of 5 mg/kg in saline were given at 7-day intervals beginning at 28 days of age. Two time courses for experimental duration were chosen. Eight dosed mice per CC line (4 males and 4 females) were killed one week after the final doxorubicin injection to study acute cardiotoxicity (acute phase). A second cohort of dosed mice (4 males and 4 females) per CC strain were killed 6 weeks after the final DOX injection to study the chronic effects of the drug (chronic or recovery phase). This regimen was duplicated for a similar cohort of control mice, who received weekly injections of saline. Mice were weighed weekly. At either 6 or 12 weeks, acute and chronic cohorts were killed, blood taken for serum biomarkers and complete blood count (CBC), and tissue collected for pathology.