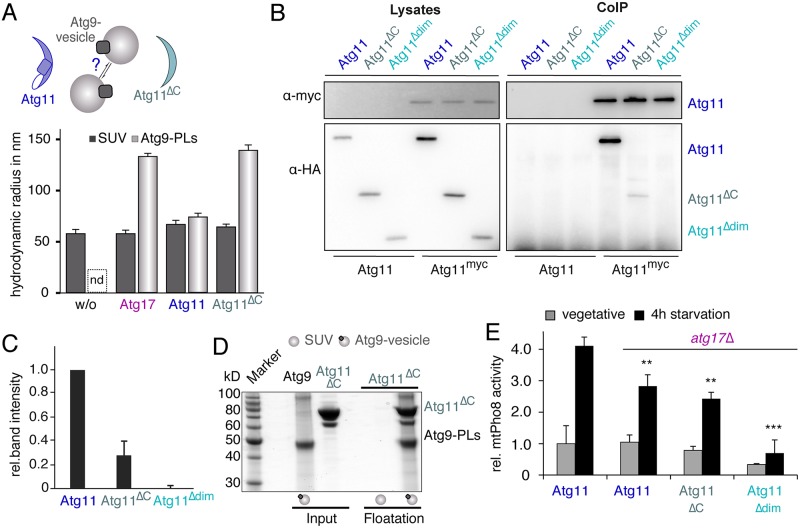
Fig 4. The N-terminus of Atg11 tethers Atg9-PLs.
(A) The RH of SUVs (dark grey bars) and Atg9-PLs (light grey bars) was determined using DLS in the absence and presence of Atg17, Atg11, and Atg11ΔC. Atg9-PLs are polydisperse in the absence of interaction partners, preventing the reliable determination of their size by light scattering. (B) The dimerization site in Atg11 was identified by IP myc-tagged full length Atg11 and co-IP of HA-tagged Atg11-variants as indicated from lysates of log-phase growing cells. Lysates of untagged Atg11 served as control. Atg11 and its fragments were detected by α-myc and α-HA immunoblots, respectively. (C) The chart shows quantification of band intensities of anti-HA blots shown in B. Values show the band intensity of co-IP Atg11 variants as a fraction of total protein levels in lysates, normalized by the amount of immunoprecipitated Atg11-myc. Values of full-length Atg11-HA were set to 1. Data are presented as mean values ± SD from three independent experiments. (D) SDS-PAGE gel with input (100% of protein used for cofloatation) and floated fractions from cofloatation assay of Atg9-PLs with Atg11ΔC. SUVs lacking Atg9core serve as control for unspecific membrane binding. (E) Mitochondrial Pho8ΔN60 (mtPho8) assay of log-phase growing and starved atg17Δ cells expressing Atg11WT, Atg11ΔC, or Atg11Δdim. Pho8-activity was corrected for total protein amount of each sample and normalized to the signal of log-phase WT cells (set to 1). Data are presented as mean values ± SD of n = 5 (A) and n = 3 (E) independent experiments. P values were calculated using a two-tailed Student t test (*P < 0.05, **P < 0.01, ***P < 0.001). DLS, dynamic light scattering; HA, hemagglutinin; IP, immunoprecipitating; nd, not determined; PL, proteoliposome; RH, hydrodynamic radius; SUV, small unilamellar vesicle; WT, wild-type.