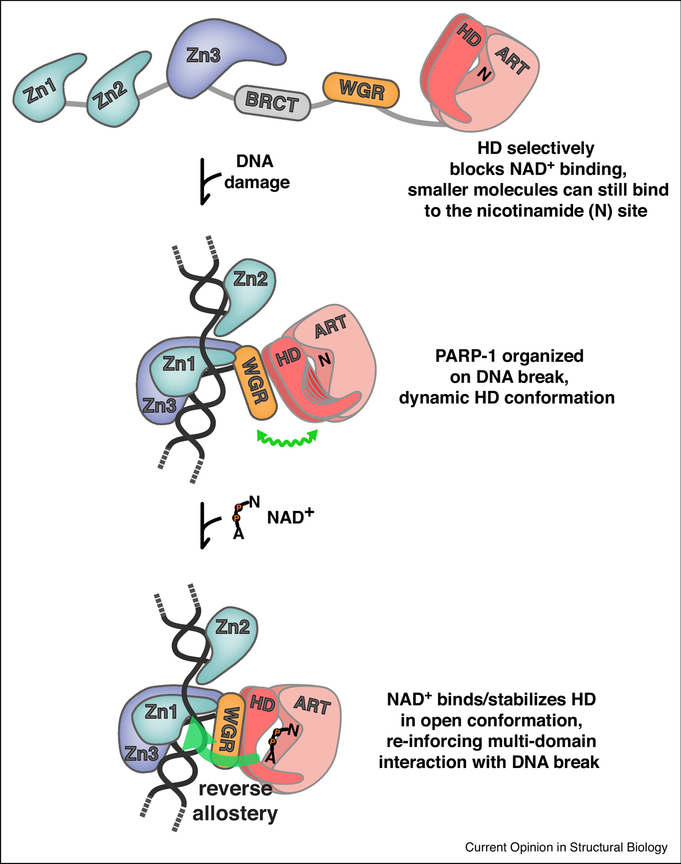
Figure 3. Allosteric regulation of PARP-1.
Regulation of NAD+ access and reverse allostery. The HD regulates PARP-1 catalytic activity through a substrate-blocking mechanism [32]. In the absence of DNA, the domains of PARP-1 exist in an open configuration. In this state, small molecules can bind to the nicotinamide site (N) of the ART; however, NAD+ binding is completely blocked. Upon detecting DNA damage, PARP-1 domains are organized around the DNA break, and an allosteric network of contacts destabilizes the HD, leading to a dynamic HD structure, illustrated as multiple potential HD conformations, and accented by a wavy green line that indicates a flexible conformation. NAD+ is now able to access the catalytic active site, engaging the nicotinamide site (N) and the adenosine site (A) that was previously blocked by the HD. NAD+ binding pushes the distribution of HD conformations toward the unfolded state, thus promoting the PARP-1 assembly of domains on DNA. Thus, NAD+ binding can influence PARP-1 interaction with DNA through a reverse allostery mechanism from catalytic active site to DNA binding domain (large green arrow).