Table 1.
Substrate Scope of Intramolecular Radical 1,5-C−H Amination of Sulfamoyl Azides via Co(II)-Based MRCa
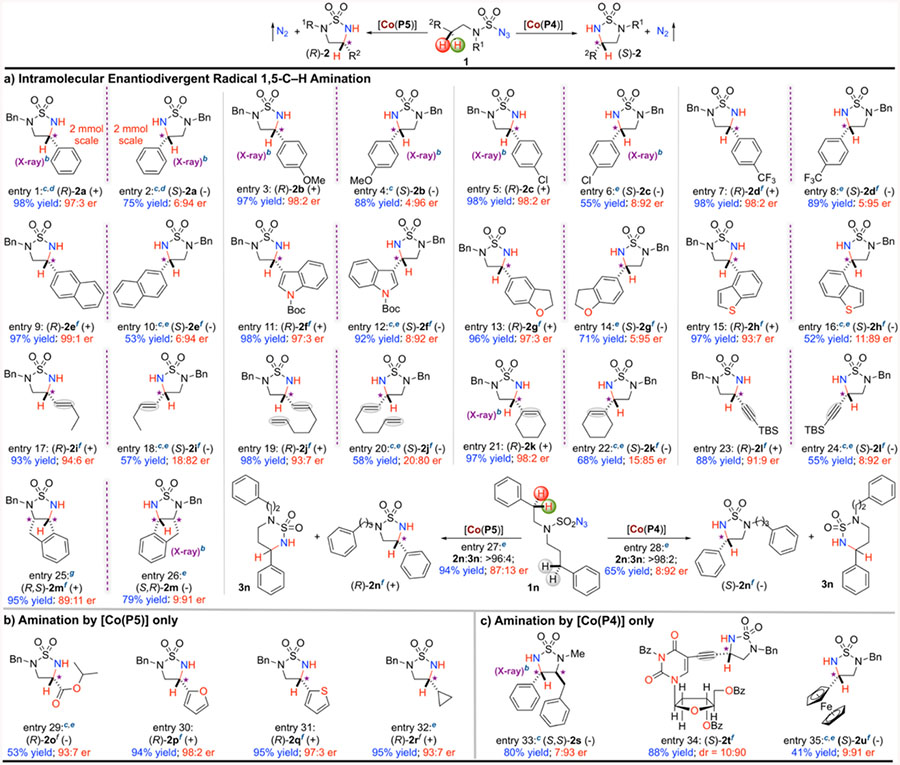
|
Reactions were performed on a 0.10 mmol scale of sulfamoyl azide 1 using 2 mol % [Co(Por*)] in 1.0 mL of methyl tert-butyl ether (MTBE) at room temperature; enantiomeric ratios (er) determined by chiral HPLC analysis.
Absolute configuration determined by X-ray crystal structural analysis.
At 40 °C.
Performed at 2.0 mmol scale with 2 mol % of [Co(Por*)].
5 mol % [Co(Por*)].
Absolute configuration assigned by analogy.
When N-Bn was replaced by N-iPr: 61% yield; 94:6 er.
