Fig. (10).
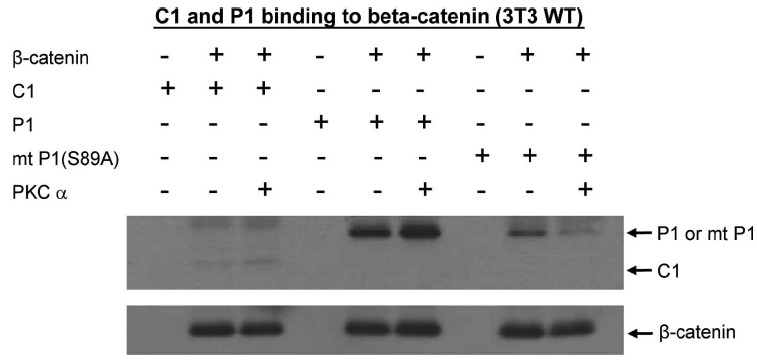
Phosphorylation of p300 Ser89 increases binding to Iý-catenin. To further investigate the biochemical effects of PKC phosphorylation of p300 on binding to Iý-catenin, His-tagged versions of the N-terminal regions (amino acid 1-111) of wild type p300 (P1), of a Ser89Ala point-mutated p300 (mt P1(S89A)), and of CBP (C1) were expressed in E. Coli and purified on a Ni-NTA column. Lysates from wild type (WT) 3T3 cells were prepared and incubated with P1, mt P1(S89A), or C1. Wild type p300 immunoprecipitated with Iý-catenin. In vitro phosphorylation of wild type p300 with PKCIñ markedly enhanced binding to Iý-catenin. Mutant p300 bound with somewhat less avidity to Iý-catenin, whereas the in vitro phosphorylated mutant p300 was dramatically less effective in binding to Iý-catenin than the phosphorylated wild type p300.
