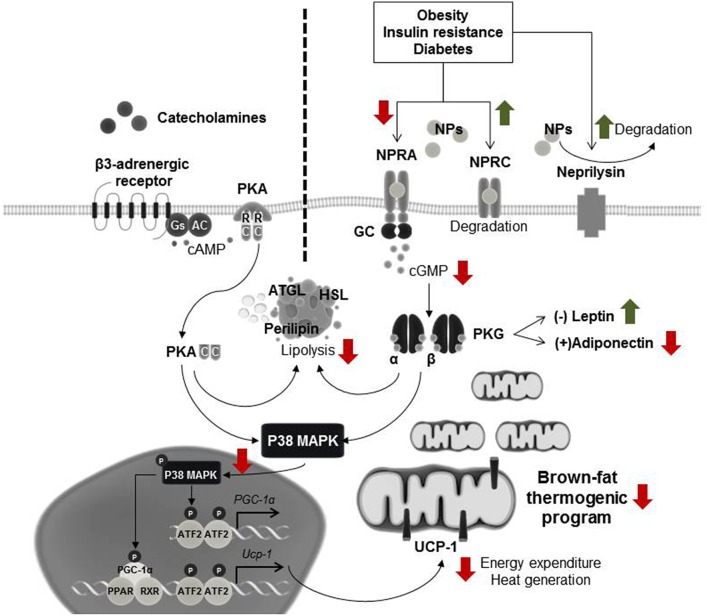
Figure 1.
Natriuretic peptide signaling in adipose tissue. Cardiac stress, such as HFpEF, induces increased natriuretic peptides levels. These natriuretic peptides bind to their receptor, natriuretic peptide active receptor (NPRA), in the adipocyte, and activate guanylyl cyclase (GC), increasing cGMP levels. Adipocytes also express natriuretic peptide clearance receptor (NPRC) that functions to remove natriuretic peptides from the circulation. The cGMP produced by NPRA-GC activates cGMP dependent protein kinase (PKG), which triggers a signaling cascade that results in enhanced lipolysis and activation of p38 mitogen-activated protein kinase (p38-MAPK), culminating in the transcription of uncoupling protein 1 (UCP-1) and inducing the brown fat thermogenic program. In parallel, other stimuli, such as cold exposure, can also induce this program via the β-adrenergic signaling pathway. Here catecholamines bind to the β-adrenergic receptor which activates adenylate cyclase (AC), producing cAMP. Binding of cAMP to the regulatory subunits (R) of cAMP-dependent protein kinase (PKA) releases its catalytic subunits (C), which also activate lipolysis and induce p38-MAPK phosphorylation. During obesity, insulin resistance and diabetes, the natriuretic peptide signaling is diminished leading to a decrease in the browning thermogenic program. Red and green arrows represent the down-regulatory or up-regulatory effects that metabolic disorders have in this signaling pathway. To date, the combined effect that obesity and HFpEF would have in adipose tissue is unknown and needs further investigation.