Abstract
Baculovirus can transduce a wide range of mammalian cells and is considered a promising gene therapy vector. However, the low transduction efficiency of baculovirus into many mammalian cells limits its practical application. Co-expressing heterologous viral glycoproteins (GPs), such as vesicular stomatitis virus G protein (VSV G), with baculovirus native envelope protein GP64 is one of the feasible strategies for improving virus transduction. Tick-borne thogotoviruses infect mammals and their GPs share sequence/structure homology and common evolutionary origins with baculovirus GP64. Herein, we tested whether thogotovirus GPs could facilitate the entry of the prototype baculovirus Autographa californica multiple multiple nucleopolyhedrovirus (AcMNPV) into mammalian cells. The gp genes of two thogotoviruses, Thogoto virus and Dhori virus, were inserted into the AcMNPV genome. Both GPs were properly expressed and incorporated into the envelope of the recombinant AcMNPVs. The transduction rates of recombinant AcMNPVs expressing the two thogotovirus GPs increased for approximately 4–12 fold compared to the wild type AcMNPV in six of the 12 tested mammalian cell lines. It seemed that thogotovirus GPs provide the recombinant AcMNPVs with different cell tropisms and showed better performance in several mammalian cells compared to VSV G incorporated AcMNPV. Further studies showed that the improved transduction was a result of augmented virus-endosome fusion and endosome escaping, rather than increased cell binding or internalization. We found the AcMNPV envelope protein GP64-mediated fusion was enhanced by the thogotovirus GPs at relatively higher pH conditions. Therefore, the thogotovirus GPs represent novel candidates to improve baculovirus-based gene delivery vectors.
Keywords: Autographa californica multiple nucleopolyhedrovirus (AcMNPV), Baculovirus, Thogotovirus, Glycoprotein, Transduction, Mammalian cells
Introduction
Baculoviruses are a group of enveloped viruses with circular double stranded DNA genomes (80–180 kb), which exclusively infect insects in nature. These viruses are widely used as insecticides and have been applied as protein expression and vaccine production vectors extensively (Contreras-Gomez et al. 2014; Dai et al. 2018; van Beek and Davis 2016). Autographa californica multiple nucleopolyhedrovirus (AcMNPV) is the prototype member of family Baculoviridae and it is one of the best studied baculoviruses. AcMNPV is able to transduce a wide range of mammalian cells and express foreign genes under a mammalian promoter in vitro and in vivo (Mansouri and Berger 2018). Therefore, baculoviruses are considered promising gene delivery and gene therapy vector.
Compared to the traditional viral gene delivery vectors, such as retroviruses, lentiviruses, adeno-associated viruses and adenoviruses, baculovirus shows many advantages. There is no pre-existing antibody against baculovirus as it doesn’t infect vertebrate in nature, and has no cytotoxic effects to mammalian cells (Ho et al. 2005; Strauss et al. 2007). With the capacity of insertion of foreign DNA fragments as large as 38 kb, baculoviruses can transduce many kinds of human cells, including both dividing and non-dividing cells, such as hepatocyte, stem cells and cancer cells, without replicating and integrating into host chromosomes (Chen et al. 2011b; Cheshenko et al. 2001; Du et al. 2010; Makkonen et al. 2015; Merrihew et al. 2001). Additionally, the construction and propagation of recombinant baculovirus is bio-safe, scalable and cost efficient by infecting its natural host insect cells (Chen et al. 2011a; Felberbaum 2015).
Although great efforts have been made to advance the baculovirus technology from bench to bedside, there are still many roadblocks stand in the way. One of the most important problems is the low transduction efficiency of baculovirus in many kinds of cells. One common strategy is to display cell specific affinity proteins or peptides, e.g. the RGD containing peptides that recognized by cell-surface integrin, on the viral envelope by fusing with the native baculoviral glycoprotein (GP), thus baculovirus transduction was significantly improved in certain cells (Ernst et al. 2006; Makela et al. 2006). An alternative method is to incorporate heterologous GPs from viruses that infect mammalian cells, such as influenza virus neuraminidase, vesicular stomatitis virus G protein (VSV G) and the GP of rabies virus (Barsoum et al. 1997; Borg et al. 2004; Tani et al. 2003). Among these, displaying VSV G is the most widely adopted, which can enhance the transduction of AcMNPV up to 15 fold (Kolangath et al. 2014). However, VSV G exhibits significant cytotoxicity in the expressed cells and results in lower baculovirus production (Kaikkonen et al. 2006). Additionally, directed mutation and screening of baculovirus GP was also used to improve the transduction in human airway epithelia (Sinn et al. 2017).
The major envelope GP of AcMNPV, GP64, is responsible for cell attachment, viral fusion and budding in insect cells (Blissard and Theilmann 2018). GP64 also contributes to the virus entry into mammalian cells (Kataoka et al. 2012). Like in insect cells, AcMNPV enters mammalian cells mainly through receptor-mediated, clathrin-dependent endocytosis pathway (Dong et al. 2010; Kataoka et al. 2012; Long et al. 2006), although macropinocytosis and clathrin-independent pathway were also reported (Kataoka et al. 2012; Laakkonen et al. 2009). Once the virions are endocytosed, the acidic environments in the endosomes will trigger the conformation change of GP64 to mediate fusion of viral envelope and endosomal membrane. Thus, the nucleocapsids are released into the cytosol and further transported to the nucleus (Liu et al. 2014). Being a class III viral fusion protein, GP64 is structurally related to the other fusion proteins in this group, including the G proteins of rhabdoviruses, the gB proteins of herpesviruses and the GPs of thogotoviruses (Mas and Melero 2013).
Thogotoviruses, belong to the Thogotovirus genus of family Orthomyxoviridae, are tick-borne viruses that infect mammals including human (Ejiri et al. 2018). The GPs of two thogotoviruses, Thogoto virus (THOV) and Dhori virus (DHOV), show no obvious similarity to that of Influenza viruses and Isavirus, but in contrast, are quite structurally related to baculovirus GP64 protein, although with a low sequence homology (~ 28% amino acid identity) (Peng et al. 2017). Interestingly, only the Group I alphabaculoviruses and a betabaculovirus possess GP64, while other baculoviruses use a distinct F protein as their envelope fusion proteins (Ardisson-Araujo et al. 2016a, b; Wang et al. 2014). It has been proposed that an ancient Group I alphabaculovirus obtained the GP64 protein from a thogotovirus-like ancestor during evolution, which may render the virus the ability to transduce a wide range of mammalian cells (Pearson and Rohrmann 2002; Peng et al. 2017).
In this study, we tested whether thogotovirus GPs will facilitate the entry of AcMNPV into mammalian cells. By incorporating the GPs of THOV and DHOV into AcMNPV envelope, the baculovirus transduction of a variety of mammalian cells was found to be significantly improved and showed different cell tropisms to the virus expressing VSV G. Further studies showed that the improvement was a result of enhanced virus-endosome fusion by the incorporation of thogotovirus GPs. Thus, the GPs of thogotoviruses provide novel candidates to improve baculovirus based gene delivery vectors.
Materials and Methods
Cell Culture and Viruses
A549, HeLa, Hep2, HepG2, HMC3, HEK 293, HUVEC, RD, SW13, SH-SY5Y, U87MG and Vero cells were obtained from the American Type Culture Collection (ATCC). A549, HeLa, HMC3, HEK 293, HUVEC, RD, SW13 and U87MG cells were cultured at 37 °C in Eagle’s Minimum Essential Medium (EMEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS; Gibco-BRL). Hep2, HepG2, SH-SY5Y and Vero cells were cultured at 37 °C in Dulbecco’s modified Eagle medium (DMEM; Gibco-BRL) supplemented with 10% FBS. Sf9 cells were cultured at 27 °C in Grace’s insect medium (Gibco-BRL) supplemented with 10% FBS. The gp64-null AcBacmid (AcBacΔgp64) was previously constructed at our laboratory (Wang et al. 2008).
Construct of Recombinant Baculoviuses
The open reading frame (ORF) of THOV GP (ThGP, GenBank accession code: NC006506) and DHOV GP (DhGP, GenBank accession code: GU969310) were chemically synthesized with a C-terminal HA tag (ThGPHA/DhGPHA). The POp166-gp64-SV40 poly(A) cassette of pFB-POp166-gp64 (Wang et al. 2008) was cut and inserted to pFastBactHTb (Invitrogen) under the polyhedrin promoter (PPolh), and the PCMV-POp166-egfp cassette of pFB-PCMV-Pop166-egfp (Dong et al. 2010) was cut and inserted downstream of POp166-gp64-SV40 poly(A) cassette, generating pFastBacHTb-BPgp64-BPegfp. Then the sequence of gp64 gene was cut off and the GPHA genes were inserted to pFastBacHTb-BPgp64-BPegfp, generating pFastBacHTb-BPGPHA-BPegfp. The donor vector pFastBacHTb-BPGPHA-BPegfp was transposed into the AcBacmid or AcBacΔgp64 (Wang et al. 2008) at the attTn7 integration site and the recombinant bacmids were named as Ac-ThGPHA, Ac-DhGPHA, AcΔgp64-ThGPHA and AcΔgp64-DhGPHA. The donor vector pFB-PCMV-Pop166-egfp was transposed into the AcBac and the recombinant bacmid was named as Ac-WT. The construction of bacmid Ac-VSVG was based on the same protocol for constructing Ac-ThGPHA and Ac-DhGPHA by inserting the ORF of VSV G into AcBacmid. The recombinant bacmids were used to transfect Sf9 cells and produced the recombinant viruses named correspondingly. The transfection and infection assay was performed as described previously (Li et al. 2018).
Immunofluorescence Microscopy
Sf9 cells were seeded into 35 mm glass bottom dish and infected by the recombinant viruses at an multiplicity of infection (MOI) of 5 (TCID50 units/cell) for 48 h. Cells were fixed by 4% paraformaldehyde, permeabilized by 0.1% Triton-X 100, washed and blocked by 5% bovine serum albumin (BSA). Then cells were incubated with the anti-HA antibody (diluted 1:1000, Sigma) in 1% BSA at room temperature for 1 h, washed and incubated with Alexa Fluor 647 goat anti-mouse antibody (Abcam) at room temperature for 1 h. Cells were washed and the cell nuclei were stained by Hoechst33258 (Beyotime). Finally, the cells were imaged by a fluorescence microscope (Deltavision softWoRx Imaging Workstation, Applied Precision) at channels Cy5 (red), FITC (green), DAPI (blue) and bright field.
Western Blot Analysis of Thogotovirus GP Incorporation in the Virions
The recombinant viruses were produced by infecting Sf9 cells for 48 h, collected before cells were lysed and purified as previously reported (Wang et al. 2010). The purified virus was suspended in PBS and prepared for the following Western blot analysis as previously reported (Zou et al. 2016). The anti-HA antibody (diluted 1:2000; Sigma) or the anti-VP39 polyclonal rabbit antibody (diluted 1:3000) (Wang et al. 2010) was used as the primary antibody and the horseradish peroxidase (HRP)-conjugated goat anti-rabbit/mouse antibody (Sigma) was used as the secondary antibody. To indicate the protein molecular weight, a PageRuler prestained protein ladder (Thermo Scientific) was used.
One-Step Growth Curve Assay
Sf9 cells were infected with recombinant virus Ac-WT, Ac-ThGPHA and Ac-DhGPHA at an MOI of 5 at 27 °C for 1 h. Then the viruses were removed and the cells were washed three times before addition of 2 mL of Grace’s medium supplemented with 10% FBS. Thirty μL of the culture were collected at 0, 24, 48, 72 and 96 h post infection (p.i.) and clarified by centrifugation at 2000 ×g for 5 min. The virus titer was determined by endpoint dilution assays.
Transducing Mammalian Cells
Based on the cell size and confluence, cells were seeded into 24 well culture plates at density of 2 × 105 cells/well (A549, HeLa, HMC3, HEK 293, RD, Hep2, HepG2 and SH-SY5Y cells) or 1 × 105 cells/well (HUVEC, SW13, U87MG and Vero cells) and cultured overnight. Cells were incubated with the virus Ac-WT, Ac-ThGPHA, Ac-DhGPHA and Ac-VSVG at an MOI of 5 or 20 at 37 °C for 1 h. Then the virus supernatant was removed and the cells were washed three times before addition of fresh culture medium. The cells were imaged by fluorescence microscopy and collected for flow cytometry (FCM) to analyze the EGFP expressing cells after culturing for 24 h.
Quantitative PCR (qPCR) Analysis of Virus Binding and Internalization
To analyze virus binding, HeLa cells seeded in 24 well culture plates (2 × 105 cells/well) were pre-chilled on ice for 30 min and then incubated with the recombinant viruses at an MOI of 5 or 20 on ice for 1 h to allow virus synchronously to bind to cells. The cells were washed with cold cell culture medium for three times before being collected for total DNA extraction. Using a pair of primers against viral gene VP80, the genomic copies of cell-bound virions were quantified by qPCR (Li et al. 2018).
For analysis of virus internalization, the viruses first bound to HeLa cells as described above. Then virus-bound cells were cultured in fresh culture medium at 37 °C for 30 min or 60 min before cells were digested by 0.5% trypsin for 10 min to remove the remaining cell-surface virions. Trypsin was neutralized by culture medium supplemented with 10% FBS and the cells were washed by cold PBS for three times. The total DNA of HeLa cells were extracted and the viral genomic copies were quantified by qPCR as described above. The quantity of internalized virions was normalized to those of cell-bound virions for each recombinant virus.
DiD Labeling of Recombinant Viruses
Sf9 cells cultured in the 75 cm2 cell culture flasks (~ 3 × 107 cells/flask) were infected by the recombinant viruses at an MOI of 2 for 24 h. Then 1,1′-Dioctadecyl-3,3,3′,3′-Tetramethylindodicarbocyanine (DiD) was added into the culture with the final concentration as 100 μmol/L. The cells were cultured for another 36 h keeping in the dark. The culture supernatants were collected, clarified by centrifugation at 2000 ×g for 5 min and filtered by 0.45 μm membrane (Millipore) to remove cell debris. Then the DiD labeled virions were pelleted from the medium by ultracentrifugation through a 20% (W/V) sucrose cushion at 18,000 rpm for 1 h at 4 °C in an SW28 rotor (Beckman Couler). The virus pellet was resuspended in Grace’s insect medium and aliquoted before store at − 80 °C.
Detection of Viral Fusion by DiD Dequenching Assay
To stain the cell cytoplasm, HeLa cells were incubated with 2 μmol/L Calcein blue, AM (Invitrogen) at 37 °C for 1 h. Then the cells were prechilled on ice for 30 min and washed two times. DiD labeled viruses were added to the cells and incubated for 1 h and unbound virions were washed away by cold cell culture medium. The cells were imaged by fluorescence microscopy immediately and finished in 5 min at channels DAPI (Calcein bule, AM), Cy5 (DiD) and bright field. The time to start imaging was set as 0 min. After imaging at 0 min, the cells were culture at 37 °C for another 30 or 60 min and imaged.
Imaging Analysis
The fluorescence intensity of DiD of each virus particle was analyzed by ImageJ software (NIH). For the fluorescence intensity of DiD at 0 min, five randomly selected fields of each recombinant virus were analyzed and the mean intensity was calculated. Two fold of the mean intensity, which beyond the max intensity at 0 min, was set as the threshold (Ith) to define dequenched DiD. To calculate the percentage of dequenched DiD at 30 and 60 min, cells was outlined based on the cytoplasm staining by Calcein blue, AM and only the fluorescent DiD dots in the cytoplasm were analyzed. The percentage of dequenched DiD was determined by dividing the number of DiD dots with intensity higher than Ith by the total number of DiD dots. For each recombinant virus at each time points, more than ten randomly selected cells were analyzed.
Neutralization Assay
The anti-GP64 mouse monoclonal antibody AcV1 is a neutralizing antibody of AcMNPV (Zhou and Blissard 2006). Fifty μL of recombinant viruses Ac-WT, Ac-ThGPHA and Ac-DhGPHA were mixed with AcV1 and diluted to a total volume of 100 μL with Grace’s insect medium. The final concentration of AcV1 was 31.3, 62.5 and 125 μg/mL. The viruses were incubated with AcV1 at 27 °C for 1 h and then added to HeLa cells at an MOI of 40 (Ac-WT) or 20 (Ac-ThGPHA and Ac-DhGPHA). After virus attachment at 37 °C for 1 h, the virus-antibody mixture was removed and cells were washed once before addition of fresh medium. Cells were cultured at 37 °C for 24 h and imaged by fluorescence microscopy.
Low pH Triggered Infection Through Direct Fusion Pathway
Low-pH triggered virus infection through direct fusion pathway was performed according to a previous report (Dong et al. 2010). HeLa cells were incubated with 40 nM bafilomycin A1 (BFA1) at 37 °C for 30 min and then were chilled on ice for 30 min. Recombinant viruses Ac-WT, Ac-ThGPHA and Ac-DhGPHA were added to the cells at an MOI of 10. After the cells were incubated with the viruses on ice for 2 h, the virus containing supernatant was removed and the cells were incubated with Grace’s medium with pH 5.0, 5.4, 5.8 or 6.2 for 5 min to trigger virus fusion at plasma membrane. The Grace’s medium was replaced with EMEM supplemented with 10% FBS and 40 nM BFA1 and the cells were cultured at 37 °C for another 4 h. Then the BFA1 containing medium was removed and the cells were washed twice before addition of fresh EMEM supplemented with 10% FBS. The cells were collected for FCM at 24 h post transdction.
Results
Construction of Recombinant Baculoviruses Expressing ThGP and DhGP
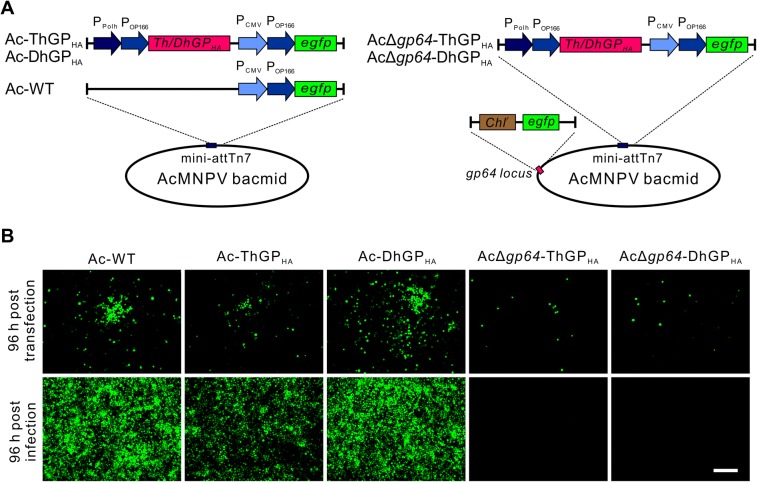
The coding sequences of ThGP and DhGP were inserted into the AcMNPV bacmid under the control of polyhedrin promoter (PPolh) and Orgyia pseudotsugata multicapsid polyhedrosis virus (OpMNPV) gp64 promoter (POp166) (Blissard and Rohrmann 1991), which would ensure high expression of the GPs in insect cells (Fig. 1A). To facilitate the detection of protein expression, a HA tag was fused with the C-terminal of GPs (GPHA). An egfp reporter gene was inserted to the virus genome under the control of promoters PCMV and POp166, which allows the EGFP expression in both mammalian- and insect cells. Although it was reported that the incorporation of ThGP could not rescue the infectivity of AcMNPV when the gp64 gene was knocked out (Lung et al. 2002), there is no study on the incorporation of DhGP in baculoviruses. In this work, both ThGP and DhGP and the reporter egfp genes were inserted to a previously constructed gp64-null bacmid AcΔgp64 (Wang et al. 2008).
Fig. 1.
Construction of recombinant baculoviruses expressing thogotovirus GPs. A The schematics of recombinant viruses. The left panel shows the schematic representation of recombinant AcMNPV bacmids carrying the reporter gene egfp and the thogotovirus GPs fused with HA tag at C-terminal. The right panel shows the baculoviruses presenting thogotovirus GPs in a gp64-null bacmid, where the gp64 gene was substituted by chloramphenicol resistance gene (Chlr) and egfp. B Transfection and infection assay of recombinant viruses. Sf9 cells were transfected by the recombinant bacmid DNA of Ac-WT, Ac-ThGPHA, Ac-DhGPHA, AcΔgp64-ThGPHA and AcΔgp64-DhGPHA. The cells were imaged at 96 h post transfection and the supernatants were collected to infect Sf9 cells and imaged at 96 h p.i. Bar, 200 μm.
Insect Sf9 cells were transfected by the recombinant bacmids. For Ac-WT, Ac-ThGPHA and Ac-DhGPHA, infectious virions were produced and resulted in significant secondary infection at 96 h post transfection (Fig. 1B). The supernatants were collected and further infection assay indicated that Ac-ThGPHA and Ac-DhGPHA showed infectivity similar to Ac-WT. For AcΔgp64-ThGPHA and AcΔgp64-DhGPHA, there were no infectious progeny virions produced as the amount of fluorescent cells did not increase even at 96 h post transfection and there was no fluorescent cells in the infection assay, suggesting that neither ThGPHA nor DhGPHA alone could rescue the infectivity of the gp64-null AcMNPV.
Thogotovirus GPs Were Incorporated into the Baculovirus Particles
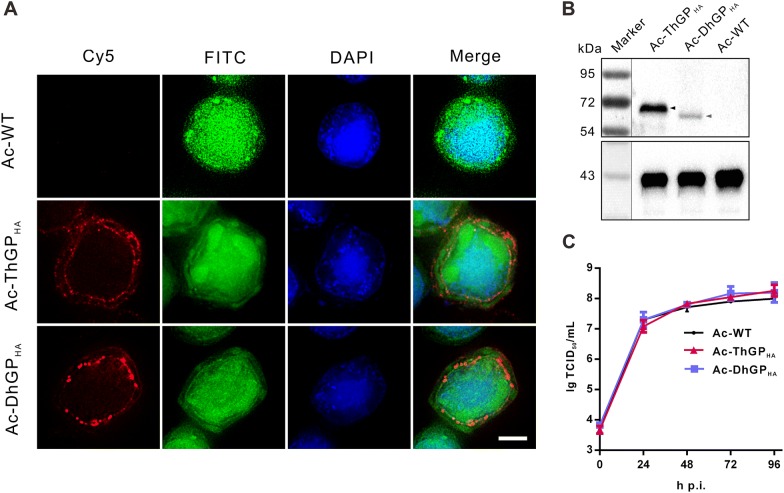
The expression and localization of ThGPHA and DhGPHA in infected Sf9 cells was detected by immunofluorescence microscopy. As shown in Fig. 2A, both ThGPHA and DhGPHA detected by anti-HA tag antibody was significantly localized in the perinuclear region and on plasma membrane, where the virions are enveloped and budded from. The incorporation of ThGPHA and DhGPHA in budded viruses was further detected by Western blot assay (Fig. 2B). Both ThGPHA and DhGPHA were detected in the virions of Ac-ThGPHA and Ac-DhGPHA, but not Ac-WT, with the molecular weight of ~ 68 kDa and ~ 63 kDa, respectively, which are in line with the previous reports that the thogotovirus GPs were expressed in insect cells (Jones et al. 1995; Peng et al. 2017).
Fig. 2.
The incorporation of thogotovirus GPs in the AcMNPV virions and its impact on virus infection. A The subcellular localization of thogotovirus GPs in infected cells. Sf9 cells were infected by Ac-WT, Ac-ThGPHA or Ac-DhGPHA and fixed at 48 h p.i. The thogotovirus GPs were detected by anti-HA antibody and Alexa flour 647 goat anti-mouse antibody (Cy5). The cell nuclei were stained by Hoechst33258 (blue). FITC channel indicates the EGFP expression in the virus infected cells. Bar, 5 μm. B Detection of the incorporation of thogotovirus GPs in the virions. The virions of recombinant viruses were purified and analyzed by Western blot assay using anti-HA antibody. The major viral capsid protein VP39 was detected by anti-VP39 antibody as control. C One-step growth curve analysis of recombinant viruses. Sf9 cells were infected by Ac-WT, Ac-ThGPHA and Ac-DhGPHA at an MOI of 5 TCID50 units/cell. The supernatants were collected at indicated time points and the virus titer was determined by endpoint dilution methods. The results are mean ± SD (n = 3 experimental replicates).
To investigate the effect of the incorporation of ThGPHA and DhGPHA on virus infection in insect cells, one-step growth curve analysis was performed. The result showed that there was no significant difference in the infectious budded virus productions between Ac-WT and Ac-DhGPHA (P > 0.05), while the production of Ac-ThGPHA increased to ~ 1.9 fold of Ac-WT (P < 0.05) (Fig. 2C).
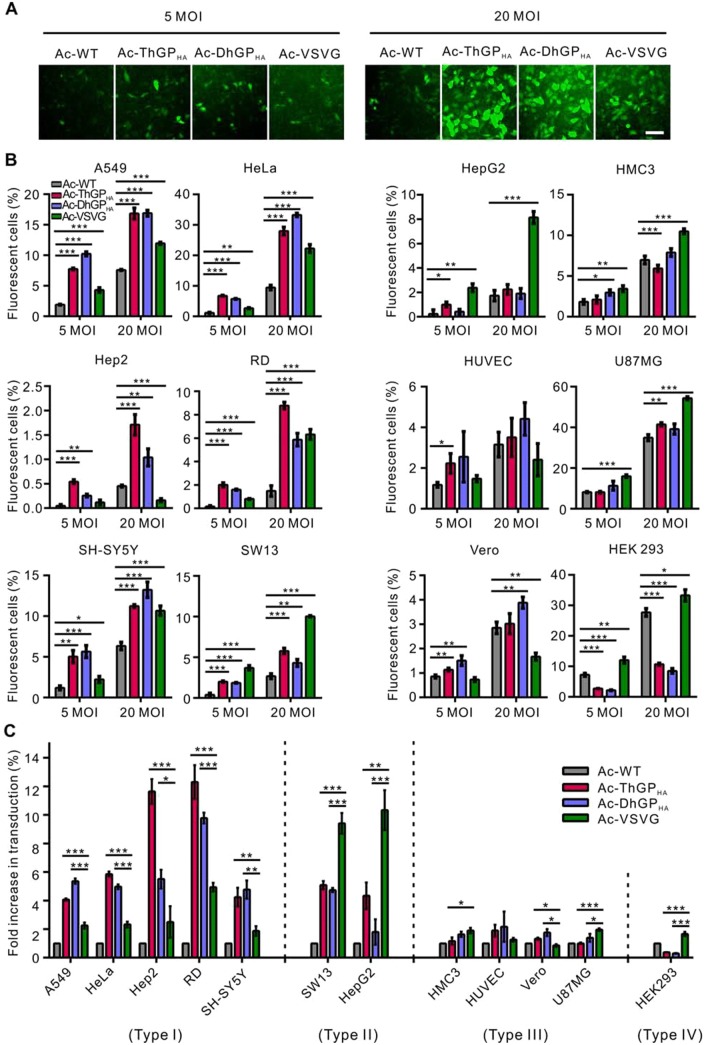
The GPs of Thogotoviruses Enhanced AcMNPV Transduction into Mammalian Cells
The transduction efficiency of Ac-WT, Ac-DhGPHA and Ac-ThGPHA was first measured in HeLa cells. Furthermore, the transduction efficiency of recombinant virus Ac-VSVG, which incorporates the GP of VSV and significantly improves the baculovirus transduction in mammalian cells was compared in parallel experiments (Barsoum et al. 1997; Tani et al. 2001). HeLa cells transduced by Ac-DhGPHA and Ac-ThGPHA at an MOI of 5 or 20 showed more EGFP positive cells and more EGFP expression than cells transduced by Ac-WT and Ac-VSVG (Fig. 3A). To further compare the transduction efficiency of Ac-WT, Ac-DhGPHA, Ac-ThGPHA and Ac-VSVG, eleven human cell lines (including HeLa) and a non-human primate cell (Vero) were transduced by the recombinant viruses and the transduction efficiency was analyzed by FCM (Fig. 3B). For Ac-DhGPHA and Ac-ThGPHA, the transduction efficiency of A549, HeLa, Hep2, RD, SH-SY5Y, and SW13 was significantly higher than those of Ac-WT at both MOI of 5 and 20, with the highest increase (~ 12 and ~ six-fold compared to Ac-WT at an MOI of 5 and 20, respectively) was observed in RD cells transduced by Ac-ThGPHA. For the other five cell lines (HepG2, HMC3, HUVEC, U87MG, and Vero cells), the transduction efficiency of Ac-DhGPHA was equal to or slightly higher (less than two-fold) than Ac-WT. The transduction of Ac-ThGPHA was similar to Ac-DhGPHA in these five cell lines, with an exception for HepG2 cells, in which the transduction efficiency was ~ four-fold of Ac-WT at an MOI of 5. Unexpectedly, the transduction efficiency of Ac-DhGPHA and Ac-ThGPHA in HEK 293 cells was notably lower than that of Ac-WT.
Fig. 3.
Incorporation of thogotovirus GPs improved the transduction of AcMNPV in mammalian cells. A HeLa cells were transduced by Ac-WT, Ac-ThGPHA, Ac-DhGPHA and Ac-VSVG at an MOI of 5 or 20 TCID50 units/cell and imaged by fluorescence microscopy at 24 h p.t. Bar, 100 μm. B Twelve mammalian cell lines were transduced by the recombinant viruses at an MOI of 5 or 20 TCID50 units/cell and analyzed by flow cytometry (FCM) at 24 h p.t. The transduction efficiency of Ac-ThGPHA and Ac-DhGPHA was significant higher than Ac-WT in six cell lines (the left panel) and was equal to, slightly higher, or even lower than Ac-WT in other six kinds of cells (the right panel). The results are mean ± SD (n = 3 experimental replicates). Significant difference relative to Ac-WT was performed by student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001. C The comparison of Ac-ThGPHA, Ac-DhGPHA and Ac-VSVG transduction into different cell lines. Data of B at an MOI of 5 TCID50 units/cell was analyzed and the fold increase of Ac-ThGPHA and Ac-DhGPHA comparing to Ac-WT was calculated. The cells were divided into four group in which the transduction efficiency of Ac-ThGPHA and Ac-DhGPHA > Ac-VSVG (type I), Ac-ThGPHA and Ac-DhGPHA < Ac-VSVG (type II), Ac-ThGPHA, Ac-DhGPHA and Ac-VSVG is equal or slightly higher than Ac-WT (type III) and Ac-ThGPHA and Ac-DhGPHA < Ac-WT (type IV). Significant difference relative to Ac-VSVG was performed by student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001.
The transduction efficiency of Ac-VSVG was significantly higher than Ac-WT in nine cell lines, with 1.6–2.5 fold increase in A549, HeLa, SH-SY5Y, HMC3, U87MG and HEK 293 cells and 5–10 fold increase in RD, SW13 and HepG2 cells at an MOI of 5. In HUVEC cells, there was no difference between Ac-VSVG and Ac-WT, while in Hep2 and Vero cells the transduction efficiency of Ac-VSVG is lower than that of Ac-WT.
Ac-DhGPHA, Ac-ThGPHA and Ac-VSVG showed significant difference in cell tropism when transducing mammalian cells as shown in Fig. 3C. In A549, HeLa, Hep2,RD and SH-SY5Y cells (type I), the transduction of Ac-DhGPHA and Ac-ThGPHA was 1.8–4.7 fold higher than Ac-VSVG. In contrast, Ac-VSVG showed (1.8–5.8 fold) higher transduction efficiency than Ac-DhGPHA and Ac-ThGPHA in SW13 and HepG2 cells (type II). Ac-DhGPHA, Ac-ThGPHA and Ac-VSVG all showed no or only slight improvement of transduction than Ac-WT in HMC3, HUVEC, Vero and U87MG cells (type III), while Ac-DhGPHA and Ac-ThGPHA even showed reduced transduction efficiency in HEK 293 cells (type IV).
The GPs of Thogotoviruses Elevated Virus-Endosome Fusion
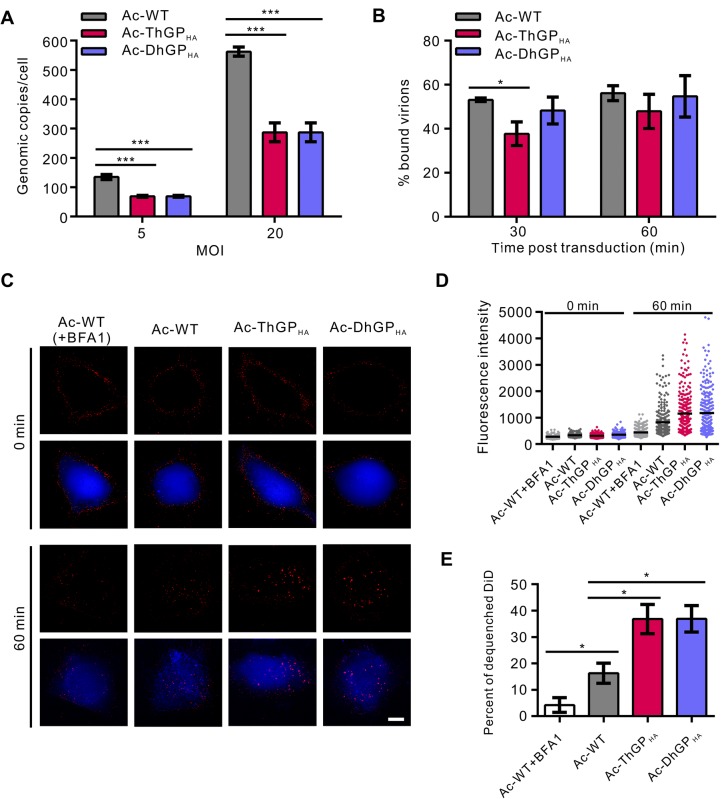
To explain why incorporation of GPs of thogotoviruses improved the baculovirus transduction, we first detected the virus binding on HeLa cells. The results showed that the binding of Ac-DhGPHA and Ac-ThGPHA was only half of Ac-WT at both MOI of 5 and 20 (Fig. 4A). Since after virus binding, the virions will be internalized by endocytosis (Kataoka et al. 2012; Long et al. 2006), we next determined whether the internalization of recombinant viruses was enhanced. The results showed that Ac-DhGPHA entered HeLa cells with similar levels to Ac-WT at 30 and 60 min post transduction (p.t.) (Figure 4B). While for Ac-ThGPHA, the virus internalization was ~ 30% lower than Ac-WT at 30 min p.t. and was equal to Ac-WT at 60 min p.t. These results suggested that the virus binding and internalization were not improved by incorporation of thogtovirus GPs.
Fig. 4.
Thogotovirus GPs enhanced the virus-endosome fusion in mammalian cells. HeLa cells were incubated with Ac-WT, Ac-ThGPHA and Ac-DhGPHA at an MOI of 5 or 20 TCID50 units/cell at 4 °C to allow the virions binding to cells. After unbound virions being washed away, the virus-bound cells were collected for DNA extraction and quantitative PCR (qPCR) to quantify the cell-bound virions (A), or cultured at 37 °C for 30 or 60 min, then cells were digested by trypsin to remove the virions bound on cell surface and the total DNA was extracted for qPCR to quantify the virions internalized into cells (B). The genomic copies were normalized to that of cell bound virions of each recombinant virus determined in A separately. The results are mean ± SD (n = 3 experimental replicates). C DiD dequeching experiment to investigate virus-endosome fusion. The cytoplasm of HeLa cells was stained by calcein AM and the cells were incubated with DiD labeled viruses at 4 °C to allow synchronous virus binding. Unbound virions were washed away and the cells were imaged immediately by fluorescence microscopy at channels DAPI (calcein AM, blue) and Cy5 (DiD, red). Then cells were cultured at 37 °C for 60 min and imaged again. For the control group (Ac-WT + BFA1), 40 nmol/L BFA1 was added in the whole experiment. Bar, 10 μm. D The analysis of fluorescence intensity of DiD. Images of C were analyzed to calculate the fluorescence intensity of each DiD labeled virions. The black lines indicate the mean fluorescence intensity. The results are represented of two randomly selected fields. E Percentage of dequenched DiDs. Images of C were analyzed to calculate the fluorescence intensity of DiD. Three fold of the mean values at 0 min was set as the threshold to define dequenched DiD and the percentages of dequenched DiD of each recombinant viruses were calculated. For each group, more than 10 randomly selected fields were analyzed. The results are mean ± SD (n = 2 independent replicates). Significant difference was analyzed by student’s t test: *P < 0.05, ** P < 0.01, *** P < 0.001.
For most of enveloped viruses, a prerequisite for successful infection is escaping from endosomes by fusion of viral envelope and endosomal membrane (Yamauchi and Helenius 2013). Here, we investigated the virus-endosome fusion of Ac-WT, Ac-ThGPHA and Ac-DhGPHA by labeling the viruses with DiD, a lipotropic dye which will dequench and show brighter fluorescence when the dye is diluted as a result of virus-endosome fusion (Spence et al. 2016). The DiD labeled viruses showed only weak fluorescence at 0 min p.t. (Figure 4C, the upper panel). While at 60 min p.t., the viruses were endocytosed into cytoplasm and fused with endosomes, thus showing brighter fluorescence, especially the viruses Ac-ThGPHA and Ac-DhGPHA (Fig. 4C, the lower panel). When cells were treated with bafliomycin A1 (BFA1), an inhibitor of endosome acidification, the virus-endosome fusion was blocked, resulting in almost no increase in DiD fluorescence intensity. The fluorescence intensity of each DiD labeled virus was analyzed (Fig. 4D). The DiD fluorescence intensity of both Ac-ThGPHA and Ac-DhGPHA is obviously higher than Ac-WT at 60 min p.t. The percentage of dequenched DiD of Ac-WT is ~ 16% at 60 min p.t. and BFA1 reduced it to ~ 4% (Fig. 4E). For both Ac-ThGPHA and Ac-DhGPHA, the percentage of dequenched DiD increased to ~ 40%. The results indicate that the AcMNPV fusion was enhanced by incorporation of the thogotovirus GPs.
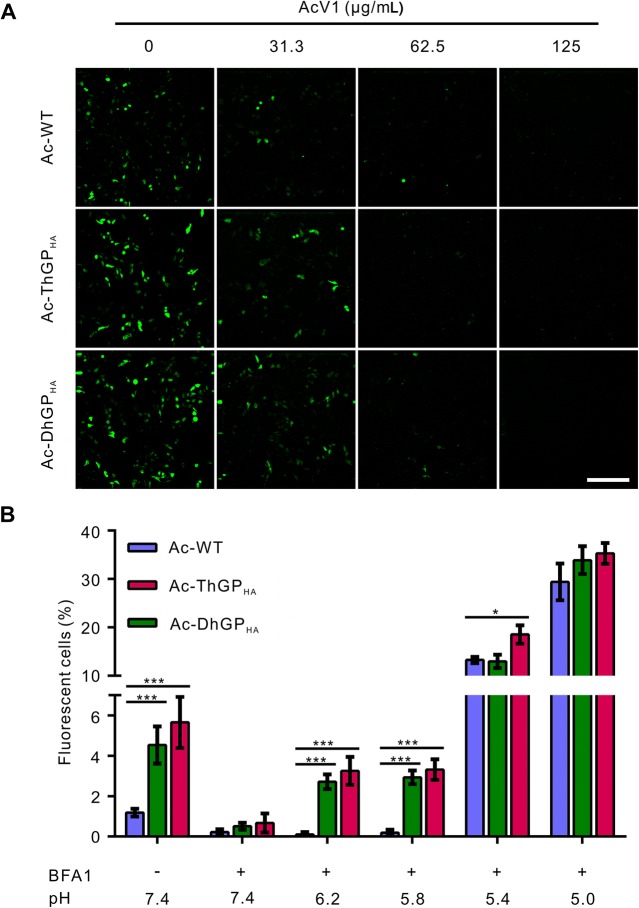
The GPs of Thogotoviruses Enhanced the GP64-Mediated Membrane Fusion at Mildly Acidic Condition
As ThGP and DhGP are responsible for the viral fusion of THOV and DHOV, respectively, we analyzed the role of ThGP and DhGP in mediating the fusion of AcMNPV. AcV1, a neutralizing antibody of GP64, was used to block the fusion activity of GP64 (Zhou and Blissard 2006). The transduction in HeLa cells of Ac-WT, Ac-ThGPHA and Ac-DhGPHA was significantly reduced by AcV1 at concentration of 31.3 μg/mL and was totally blocked at 125 μg/mL (Fig. 5A). As AcV1 inhibits the fusion activity, but not the cell binding ability of GP64, the results suggested that the GPs of thogotoviruses could not mediate the fusion of AcMNPV independently.
Fig. 5.
Thogotovirus GPs enhanced the GP64-mediated membrane fusion at higher pH conditions. A The transduction of recombinant viruses were blocked by neutralizing antibody against the fusion activity of GP64. Ac-WT, Ac-ThGPHA and Ac-DhGPHA were incubated with neutralizing antibody AcV1 at indicated concentration and then used to transduce HeLa cells. The cells were imaged for EGFP expressing at 24 h p.t. The results are represent of two independent experiments. Bar, 200 μm. B Thogotovirus GPs enhanced the viral fusion at relative high pH condition. HeLa cells were incubated with the recombinant virus at 4 °C to allow virus binding. The virion-bound cells were treated by medium with indicated pH to trigger virus-plasma membrane fusion. Forty nmol/L BFA1 was used to block virus entry through the endocytosis pathway. Cells were analyzed by FCM at 24 h p.t. for EGFP expression. The results are mean ± SD (n = 3 experimental replicates). Significant difference relative to Ac-WT was performed by student’s t test: *P < 0.05; **P < 0.01; ***P < 0.001.
GP64 is a low-pH dependent viral fusion protein, with optimal pH lower than 5.5 (Blissard and Wenz 1992). It is previously reported that a short time treatment with low-pH (pH 4.8) after binding of AcMNPV to mammalian cells can trigger virus fusion at plasma membrane, resulting in high efficient baculovirus transduction of mammalian cells (Dong et al. 2010). We tested whether the GP64 mediated fusion was enhanced by the GPs of thogotoviruses by a similar experiment. When the cellular endocytosis pathway was blocked by BFA1, a short time treatment of pH 5.4 and 5.0 increased the transduction rate of Ac-WT from 0.2% (pH 7.4 + BFA1) to 13% and 29%, respectively, and the transduction rate of Ac-ThGPHA and Ac-DhGPHA was equal to or slightly higher than Ac-WT (Fig. 5B). Interestingly, when treated with higher pH condition (pH 6.2 and 5.8), there was no increase in transduction of Ac-WT, while the transduction of Ac-ThGPHA and Ac-DhGPHA was increased from ~ 0.5% (pH 7.4 + BFA1) to ~ 3%. The results indicates that the GPs of thogotoviruses enhanced the GP64 mediated membrane fusion at mildly acidic condition with pH higher than 5.4.
Discussion
AcMNPV is a promising gene therapy vector as it can transduce a wide variety of human cells with advantages over the traditional viral vectors (Hu 2010). However, the low transduction efficiency of most mammalian cells is one of the major obstacles that limits the clinical application of AcMNPV (Mansouri and Berger 2018). Although a short time low pH treatment can greatly improve the virus transduction, it is not exercisable for in vivo application (Dong et al. 2010). Incorporation of GPs from other viruses into the virions of AcMNPV seems to be the most commonly used and effective method to enhance baculovirus transduction both in vitro and in vivo (Ono et al. 2018). In this study, the GPs of THOV and DHOV were successfully incorporated into viral envelope of AcMNPV and their transduction efficiency was determined in a wide range of mammalian cell lines with comparison of VSV G incorporated AcMNPV.
ThGP and DhGP improved the baculovirus transduction efficiency from 4 to 12 fold compared to Ac-WT in six of the 12 selected cell lines we tested at an MOI of 5 and showed tropism of wide range of cell types, including the epithelial cells (A549, HeLa and Hep2), muscle cells (RD), bone marrow neuroblast cells (SH-SY5Y) and adrenal adenocarcinoma cells (SW13). VSV G has been widely used to enhance baculovirus transduction efficiency and expand the cell tropism (Barsoum et al. 1997; Kolangath et al. 2014). Here, we found that the transduction efficiency of Ac-VSVG is higher than Ac-WT in nine cell lines, but increased by more than four-fold only in three of them at an MOI of 5. The transduction of AcMNPV in five cell lines, including A549, HeLa, RD, SW13 and SH-SY5Y, was significantly improved by both thogotovirus GPs and VSV G at an MOI of 5. Among these, the transduction efficiency of Ac-ThGPHA and Ac-DhGPHA is higher than Ac-VSVG in four kinds of cells (A549, HeLa, RD and SH-SY5Y) and lower in one cell line (SW13) (Fig. 3B). Additionally, the baculovirus transduction in Hep2 cells was significantly improved only by thogotovirus GPs, but not by VSV G. The results indicate that thogotovirus GPs and VSV G render baculovirus different cell tropism, and thogotovirus GPs seems to more effective in increasing baculovirus transduction in some cell lines compared to VSV G, such as A549, HeLa, Hep2, RD, and SH-SY5Y cells; Whereas VSV G exihibt better performance than thogotovirus GPs in SW13, HMC3, HepG2, and U87MG cells. Therefore, recombinant virus with different GPs should be considered for improving baculovirus transduction into different human cells.
To elucidate the mechanism that how GPs of thogotoviruses enhance baculovirus transduction in mammalian cells, virus binding was first detected. Unexpectedly, the binding of Ac-ThGPHA and Ac-DhGPHA on HeLa cells was only about 50% to that of Ac-WT. This may be due to the incorporation of ThGP/DhGP impaired the amounts of GP64 assembled on the viral envelope or interfered the receptor binding activity of GP64. Following cell attachment, AcMNPV will be internalized into cells through clathrin-mediated endocytosis pathway (Kataoka et al. 2012). The results showed that the internalization of Ac-ThGPHA and Ac-DhGPHA was almost equivalent to Ac-WT at 60 min p.t. As the ThGP/DhGP did not enhance virus binding and internalization, it was speculated that the endosomal escaping was augmented. This idea was confirmed by the DiD dequenching experiments that indicated the virus-endosome fusion. The percentages of dequenched DiD of Ac-ThGPHA and Ac-DhGPHA were 2.3 fold of Ac-WT at 60 min p.t., suggesting more efficient viral fusion and endosome escaping (Fig. 4E).
However, when the fusion activity of GP64 was blocked, Ac-ThGPHA and Ac-DhGPHA failed to transduce mammalian cells, indicating that ThGP and DhGP could not mediate the viral fusion of baculoviruses independently. A possible explanation is that the incomplete glycosylation of the GPs impaired the fusion activity, as the molecular weight of ThGP expressed in insect cells was ~ 68 kDa (Fig. 2B) and is significantly smaller than that expressed in mammalian cells (~ 75 kDa) (Agustin et al. 1992). Interestingly, the GP64 mediated fusion was enhanced by ThGP and DhGP at pH 5.8 and 6.2. It was reported that a less than 21-amino-acid ectodomain together with the transmembrane and cytoplasmic tail domain of VSV G could augment the membrane fusion activity of some heterologous viral envelope proteins, including AcMNPV GP64 (Jeetendra et al. 2002; Kaikkonen et al. 2006). Both THOV and DHOV possess only one envelope GP and the conformational change of the GP was observed upon low pH treatment, with the highest haemolysis activity at pH 6.0–6.2 and cell–cell fusion at pH 6.0 (Agustin et al. 1992; Peng et al. 2017). Although pH < 5.5 is essential for the fusion activity of GP64 measured by cell–cell fusion, weak fusion activity was observed by virus-liposome fusion at pH 5.5–6.5 (Kamiya et al. 2010). It was reported that the baculovirus transduction in HeLa cells dependent on early endosomes but not late endosomes (Liu et al., 2014), of which the pH are 6.8–5.9 and 6.0–4.9, respectively (Huotari and Helenius 2011). We speculate that the GP64 mediated virus-endosome fusion in the mildly acidic early endosomes of mammalian cells is augmented by ThGP and DhGP.
In conclusion, the GPs of two thogotoviruses were expressed and incorporated into the virions of AcMNV. By incorporating these GPs into AcMNPV envelope, the baculovirus transduction in a variety of human cells was significantly improved. The improvement of transduction is probably a result of enhanced GP64 mediated virus-endosome fusion by the incorporation of thogotovirus GPs. These viruses showed higher transduction efficiency than Ac-VSVG in many cells, providing us more choices to improve baculovirus transduction for gene delivery.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (Grant Nos. 31370191 and 31621061), Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB11030400) and the National Key R&D Program of China (Grant No. 2018YFA0507200). We thank Dr. Ding Gao, Ms. Juan Min, Mr. He Zhao and Ms. Li Li from the Core Facility and Technical Support facility of the Wuhan Institute of Virology for technical assistance.
Author Contributions
LH, YL, MW and HW designed the research; LH and YL performed research and analyzed data; LH and MW wrote the paper; FD, ZH and HW edited and commented on the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare no competing interests.
Animal and Human Rights Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Hualin Wang, Phone: 86-27-87199353, Email: h.wang@wh.iov.cn.
Manli Wang, Phone: +86-27-87197340, Email: wangml@wh.iov.cn.
References
- Agustin P, Linda D, Patricia N. Inentification of viral structrual polypeptides of Thogoto virus (a tick-borne orthomyxo-like virus) and functions associated with the glycoprotein. J Gen Virol. 1992;73:2823–2830. doi: 10.1099/0022-1317-73-11-2823. [DOI] [PubMed] [Google Scholar]
- Ardisson-Araujo DM, Melo FL, Clem RJ, Wolff JL, Ribeiro BM. A betabaculovirus-encoded gp64 homolog codes for a functional envelope fusion protein. J Virol. 2016;90:1668–1672. doi: 10.1128/JVI.02491-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardisson-Araujo DM, Pereira BT, Melo Fl, Ribeiro BM, Bao SN, de AZPM, Moscardi F, Kitajima EW, Sosa-Gomez DR. A betabaculovirus encoding a gp64 homolog. BMC Genom. 2016;17:94. doi: 10.1186/s12864-016-2408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum J, Brown R, McKee M, Boyce FM. Efficient transduction of mammalian cells by a recombinant baculovirus having the vesicular stomatitis virus G glycoprotein. Hum Gene Ther. 1997;8:2011–2018. doi: 10.1089/hum.1997.8.17-2011. [DOI] [PubMed] [Google Scholar]
- Blissard GW, Rohrmann GF. Baculovirus gp64 gene expression: analysis of sequences modulating early transcription and transactivation by IE1. J Virol. 1991;65:5820–5827. doi: 10.1128/jvi.65.11.5820-5827.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard GW, Theilmann DA. Baculovirus entry and egress from insect cells. Annu Rev Virol. 2018;5:9.1–9.27. doi: 10.1146/annurev-virology-092917-043356. [DOI] [PubMed] [Google Scholar]
- Blissard GW, Wenz JR. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg J, Nevsten P, Wallenberg R, Stenstrom M, Cardell S, Falkenberg C, Holm C. Amino-terminal anchored surface display in insect cells and budded baculovirus using the amino-terminal end of neuraminidase. J Biotechnol. 2004;114:21–30. doi: 10.1016/j.jbiotec.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Chen CY, Lin CY, Chen GY, Hu YC. Baculovirus as a gene delivery vector: recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol Adv. 2011;29:618–631. doi: 10.1016/j.biotechadv.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wu HH, Chen CP, Chern SR, Hwang SM, Huang SF, Lo WH, Chen GY, Hu YC. Biosafety assessment of human mesenchymal stem cells engineered by hybrid baculovirus vectors. Mol Pharm. 2011;8:1505–1514. doi: 10.1021/mp100368d. [DOI] [PubMed] [Google Scholar]
- Cheshenko N, Krougliak N, Eisensmith RC, Krougliak VA. A novel system for the production of fully deleted adenovirus vectors that does not require helper adenovirus. Gene Ther. 2001;8:846–854. doi: 10.1038/sj.gt.3301459. [DOI] [PubMed] [Google Scholar]
- Contreras-Gomez A, Sanchez-Miron A, Garcia-Camacho F, Molina-Grima E, Chisti Y. Protein production using the baculovirus-insect cell expression system. Biotechnol Prog. 2014;30:1–18. doi: 10.1002/btpr.1842. [DOI] [PubMed] [Google Scholar]
- Dai S, Zhang T, Zhang Y, Wang H, Deng F. Zika virus baculovirus-expressed virus-like particles induce neutralizing antibodies in mice. Virol Sin. 2018;33:213–226. doi: 10.1007/s12250-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Wang M, Qiu Z, Deng F, Vlak JM, Hu Z, Wang H. Autographa californica multicapsid nucleopolyhedrovirus efficiently infects Sf9 cells and transduces mammalian cells via direct fusion with the plasma membrane at low pH. J Virol. 2010;84:5351–5359. doi: 10.1128/JVI.02517-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zeng J, Zhao Y, Boulaire J, Wang S. The combined use of viral transcriptional and post-transcriptional regulatory elements to improve baculovirus-mediated transient gene expression in human embryonic stem cells. J Biosci Bioeng. 2010;109:1–8. doi: 10.1016/j.jbiosc.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Ejiri H, Lim CK, Isawa H, Fujita R, Murota K, Sato T, Kobayashi D, Kan M, Hattori M, Kimura T. Characterization of a novel thogotovirus isolated from Amblyomma testudinarium ticks in Ehime, Japan: a significant phylogenetic relationship to Bourbon virus. Virus Res. 2018;249:57–65. doi: 10.1016/j.virusres.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Ernst W, Schinko T, Spenger A, Oker-Blom C, Grabherr R. Improving baculovirus transduction of mammalian cells by surface display of a RGD-motif. J Biotechnol. 2006;126:237–240. doi: 10.1016/j.jbiotec.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Felberbaum RS. The baculovirus expression vector system: a commercial manufacturing platform for viral vaccines and gene therapy vectors. Biotechnol J. 2015;10:702–714. doi: 10.1002/biot.201400438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho YC, Chung YC, Hwang SM, Wang KC, Hu YC. Transgene expression and differentiation of baculovirus-transduced human mesenchymal stem cells. J Gene Med. 2005;7:860–868. doi: 10.1002/jgm.729. [DOI] [PubMed] [Google Scholar]
- Hu YC. Baculovirus: a promising vector for gene therapy? Curr Gene Ther. 2010;10:167. doi: 10.2174/156652310791321260. [DOI] [PubMed] [Google Scholar]
- Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeetendra E, Robison CS, Albritton LM, Whitt MA. The membrane-proximal domain of vesicular stomatitis virus G protein functions as a membrane fusion potentiator and can induce hemifusion. J Virol. 2002;76:12300–12311. doi: 10.1128/JVI.76.23.12300-12311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LD, Morse MA, Marriott AC, Nuttall PA. Immune protection conferred by the baculovirus-related glycoprotein of Thogoto virus (Orthomyxoviridae) Virology. 1995;213:249–253. doi: 10.1006/viro.1995.1566. [DOI] [PubMed] [Google Scholar]
- Kaikkonen MU, Raty JK, Airenne KJ, Wirth T, Heikura T, Yla-Herttuala S. Truncated vesicular stomatitis virus G protein improves baculovirus transduction efficiency in vitro and in vivo. Gene Ther. 2006;13:304–312. doi: 10.1038/sj.gt.3302657. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Kobayashi J, Yoshimura T, Tsumoto K. Confocal microscopic observation of fusion between baculovirus budded virus envelopes and single giant unilamellar vesicles. Biochim Biophys Acta. 2010;1798:1625–1631. doi: 10.1016/j.bbamem.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Kataoka C, Kaname Y, Taguwa S, Abe T, Fukuhara T, Tani H, Moriishi K, Matsuura Y. Baculovirus GP64-mediated entry into mammalian cells. J Virol. 2012;86:2610–2620. doi: 10.1128/JVI.06704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolangath SM, Basagoudanavar SH, Hosamani M, Saravanan P, Tamil Selvan RP. Baculovirus mediated transduction: analysis of vesicular stomatitis virus glycoprotein pseudotyping. Virusdisease. 2014;25:441–446. doi: 10.1007/s13337-014-0229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakkonen JP, Makela AR, Kakkonen E, Turkki P, Kukkonen S, Peranen J, Yla-Herttuala S, Airenne KJ, Oker-Blom C, Vihinen-Ranta Clathrin-independent entry of baculovirus triggers uptake of E. coli in non-phagocytic human cells. PLoS ONE. 2009;4:e5093. doi: 10.1371/journal.pone.0005093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shen S, Hu L, Deng F, Vlak JM, Hu Z, Wang H, Wang M. The functional oligomeric state of tegument protein GP41 is essential for baculovirus budded virion and occlusion-derived virion assembly. J Virol. 2018;92:e02083–02017. doi: 10.1128/JVI.02083-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Joo KI, Lei Y, Wang P. Visualization of intracellular pathways of engineered baculovirus in mammalian cells. Virus Res. 2014;181:81–91. doi: 10.1016/j.virusres.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long G, Pan X, Kormelink R, Vlak JM. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J Virol. 2006;80:8830–8833. doi: 10.1128/JVI.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung O, Westenberg M, Vlak JM, Zuidema D, Blissard GW. Pseudotyping Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV): F proteins from group II NPVs are functionally analogous to AcMNPV GP64. J Virol. 2002;76:5729–5736. doi: 10.1128/JVI.76.11.5729-5736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela AR, Matilainen H, White DJ, Ruoslahti E, Oker-Blom C. Enhanced baculovirus-mediated transduction of human cancer cells by tumor-homing peptides. J Virol. 2006;80:6603–6611. doi: 10.1128/JVI.00528-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen KE, Airenne K, Yla-Herttulala S. Baculovirus-mediated gene delivery and RNAi applications. Viruses. 2015;7:2099–2125. doi: 10.3390/v7042099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri M, Berger P. Baculovirus for gene delivery to mammalian cells: past, present and future. Plasmid. 2018;98:1–7. doi: 10.1016/j.plasmid.2018.05.002. [DOI] [PubMed] [Google Scholar]
- Mas V, Melero JA. Entry of enveloped viruses into host cells: membrane fusion. Subcell Biochem. 2013;68:467–487. doi: 10.1007/978-94-007-6552-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrihew RV, Clay WC, Condreay JP, Witherspoon SM, Dallas WS, Kost TA. Chromosomal integration of transduced recombinant baculovirus DNA in mammalian cells. J Virol. 2001;75:903–909. doi: 10.1128/JVI.75.2.903-909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono C, Okamoto T, Abe T, Matsuura Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses. 2018;10:510. doi: 10.3390/v10090510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson MN, Rohrmann GF. Transfer, Incorporation, and substitution of envelope fusion proteins among members of the baculoviridae, orthomyxoviridae, and metaviridae (insect retrovirus) families. J Virol. 2002;76:5301–5304. doi: 10.1128/JVI.76.11.5301-5304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, Zhang S, Cui Y, Shi Y, Gao GF, Qi J. Structures of human-infecting Thogotovirus fusogens support a common ancestor with insect baculovirus. Proc Natl Acad Sci USA. 2017;114:E8905–E8912. doi: 10.1073/pnas.1706125114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn PL, Hwang BY, Li N, Ortiz JLS, Shirazi E, Parekh KR, Cooney AL, Schaffer DV, McCray PB., Jr Novel GP64 envelope variants for improved delivery to human airway epithelial cells. Gene Ther. 2017;24:674–679. doi: 10.1038/gt.2017.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JS, Krause TB, Mittler E, Jangra RK, Chandran K. Direct visualization of ebola virus fusion triggering in the endocytic pathway. MBio. 2016;7:e01857–e01815. doi: 10.1128/mBio.01857-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, Huser A, Ni S, Tuve S, Kiviat N, Sow PS, Hofmann C, Lieber A. Baculovirus-based vaccination vectors allow for efficient induction of immune responses against plasmodium falciparum circumsporozoite protein. Mol Ther. 2007;15:193–202. doi: 10.1038/sj.mt.6300008. [DOI] [PubMed] [Google Scholar]
- Tani H, Nishijima M, Ushijima H, Miyamura T, Matsuura Y. Characterization of cell-surface determinants important for baculovirus infection. Virology. 2001;279:343–353. doi: 10.1006/viro.2000.0699. [DOI] [PubMed] [Google Scholar]
- Tani H, et al. in vitro and in vivo gene delivery by recombinant baculoviruses. J Virol. 2003;77:9799–9808. doi: 10.1128/JVI.77.18.9799-9808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beek N, Davis DC. Baculovirus insecticide production in insect larvae. Methods Mol Biol. 2016;1350:393–405. doi: 10.1007/978-1-4939-3043-2_20. [DOI] [PubMed] [Google Scholar]
- Wang M, Tan Y, Yin F, Deng F, Vlak JM, Hu Z, Wang H. The F-like protein Ac23 enhances the infectivity of the budded virus of gp64-null Autographa californica multinucleocapsid nucleopolyhedrovirus pseudotyped with baculovirus envelope fusion protein F. J Virol. 2008;82:9800–9804. doi: 10.1128/JVI.00759-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Deng F, Hou D, Zhao Y, Guo L, Wang H, Hu Z. Proteomics of the Autographa californica nucleopolyhedrovirus budded virions. J Virol. 2010;84:7233–7242. doi: 10.1128/JVI.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang J, Yin F, Tan Y, Deng F, Chen X, Jehle JA, Vlak JM, Hu Z, Wang H. Unraveling the entry mechanism of baculoviruses and its evolutionary implications. J Virol. 2014;88:2301–2311. doi: 10.1128/JVI.03204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi Y, Helenius A. Virus entry at a glance. J Cell Sci. 2013;126:1289–1295. doi: 10.1242/jcs.119685. [DOI] [PubMed] [Google Scholar]
- Zhou J, Blissard GW. Mapping the conformational epitope of a neutralizing antibody (AcV1) directed against the AcMNPV GP64 protein. Virology. 2006;352:427–437. doi: 10.1016/j.virol.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Liu J, Wang Z, Deng F, Wang H, Hu Z, Wang M, Zhang T. Characterization of two monoclonal antibodies, 38F10 and 44D11, against the major envelope fusion protein of Helicoverpa armigera nucleopolyhedrovirus. Virol Sin. 2016;31:490–499. doi: 10.1007/s12250-016-3831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]