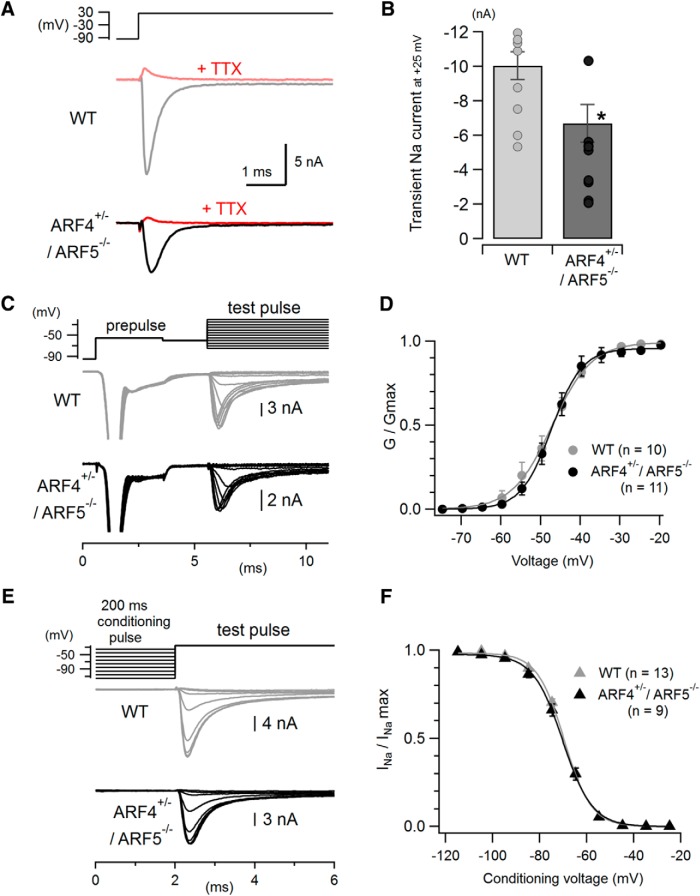
Figure 5.
Reduced fast transient Na+ currents with no change in activation or inactivation properties in cerebellar ARF4+/−/ARF5−/− PCs. A, Representative traces of fast transient Na+ currents evoked by a depolarizing voltage pulse from −95 mV to +25 mV in WT (gray) and ARF4+/−/ARF5−/− (black) PCs. The recorded currents were abolished by extracellular application of 1 μm TTX, a Na+ channel blocker (red). B, Pooled data of peak amplitudes of the fast transient Na+ currents recorded at +25 mV as described in A. Bar graphs show mean values, and symbols represent individual data points. *p < 0.05, Student's t test. C, Representative traces used in the analysis of voltage dependence of transient Na+ current activation (test pulses ranging from −75 mV to −15 mV in 5 mV increments) recorded from PCs in cerebellar slices. A pre-pulse of −55 mV (subthreshold for proximal Na+ channels) was applied before test pulses to elicit unclamped axonal Na+ spikes and inactivate distal Na+ channels, leaving well controlled proximal Na+ channels available for the upcoming test pulses. D, Normalized conductance–voltage relations for the fast transient Na+ currents in response to the test pulses shown in C in WT (gray) and ARF4+/−/ARF5−/− (black) PCs. The activation (G/Gmax) curves were fit with Boltzmann functions (see Materials and Methods). The voltage dependency of the transient Na+ current activation is similar between WT and ARF4+/−/ARF5−/− PCs. E, Representative traces used in the analysis of voltage-dependent steady-state inactivation of Na+ currents. Conditioning voltage pulses (200 ms, ranging from −115 mV to −25 mV in 10 mV steps) were applied to determine the voltage dependence of steady-state inactivation before a 10 ms test pulse to −25 mV. F, Steady-state inactivation curve for the fast transient Na+ currents. Normalized Na+ currents (INa/INamax) evoked by test pulses were plotted against conditioning voltages. Data were fit with Boltzmann functions (see Materials and Methods).