Figure 4.
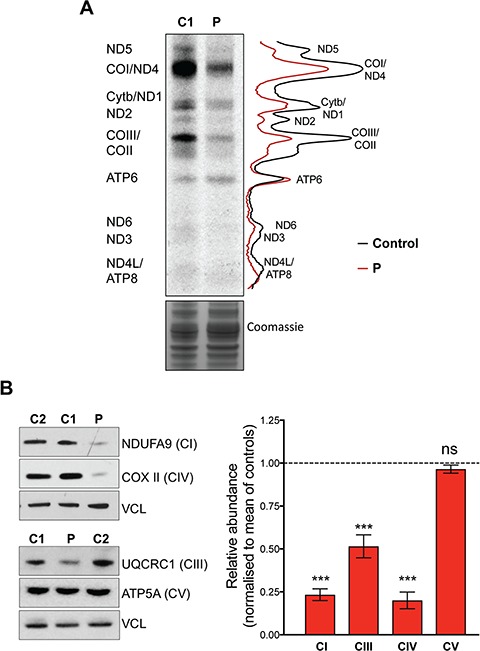
MRPS25 mutation reduced mitochondrial translation and causes a combined OXPHOS defects in the affected patient. (A) De novo mitochondrial protein synthesis measured by 35S-methionine incorporation in control (C1) and patient (P) fibroblasts. The gel image is flanked by polypeptide assignments to the left and plot profiles showing the pixel intensities for the control (black line) and patient (P–red line) are shown on the right. Coomassie staining of total protein was used as loading control. (B) To the left, representative immunoblots of OXPHOS components of complex I (NDUFA9), complex III (UQCRC1), complex IV (COXII) and complex V (ATP5A). Levels of vinculin (VCL) were used as indicators of protein loading. To the right, a chart indicating the abundance of the respiratory chain proteins in the patient fibroblasts compared to controls (Fiji ImageJ densitometric analysis). The data are the mean ± standard error of the mean of n ≥ 3 independent experiments. Probability was determined using Welch’s t-test (ns = not significant, P > 0.05; ***P < 0.001).
