Abstract
Background
Ventilation practice may be affected by economic variations, which might result in different outcomes to mechanically ventilated patients. We aimed to investigate the important effect of economic variations in patients with mechanical ventilation (MV) in China.
Methods
We carried out a national prospective multicentre cross-sectional observational study over 1 month of all patients receiving invasive MV for more than 24 hours in 20 intensive care units (ICUs), including patient characteristics, practice of MV, weaning modalities and outcomes, including probability of weaning and survival. Based on the 2012 World Bank classification of counties, patients were divided into high-income and middle-income groups according to gross domestic product per capita in their province of origin.
Results
Of the 483 patients enrolled, 291 (60.2%) were from high-income provinces and 192 (39.8%) were from middle-income provinces. Tidal volume, peak pressure, plateau and driving pressure were significantly lower, and the proportion of patients receiving protective ventilation (71.1% vs. 59.9%, P=0.014) was significantly higher in the high-income group than in the middle-income group. The probability of weaning within 28 days was significantly greater in the high-income group than in the middle-income group (P=0.046). Patients in the high-income group had significantly higher median numbers of ventilator-free days within 14 and 28 days than those in the middle-income group (P<0.05). Although the patients did not differ in terms of their demographics, survival within 28 days was significantly higher in the high-income group than in the middle-income group (P=0.025). Driving pressure, positive end-expiratory pressure and spontaneous breathing trial were independently associated with hospital mortality.
Conclusions
Important economic differences exist in the management of MV and patient outcomes. Higher income is associated with a higher proportion of protective ventilation, lower driving pressure, shorter weaning and better survival in mechanically ventilated patients in China.
Keywords: Respiration, artificial; economics; China; ventilator weaning; in-hospital mortalities
Introduction
Mechanical ventilation (MV) is the cornerstone in the supportive management of patients with acute respiratory failure and has been reported to be used in about one-third of patients in intensive care units (1-3). MV did not become widely available in China until the 1980s. Meanwhile, with the rapid development of the country’s economy, healthcare system and international communication, the use and patient outcomes of patients with MV have been deeply influenced. However, an imbalanced economic development between the southeast coast and other regions has led to the imbalanced development of healthcare. Important economic variations in patterns of care have been described for the use of interventions such as blood transfusion (4), amputation (5), aneurysm repair (6), carotid revascularization (7), and outcomes of acute respiratory distress syndrome (ARDS) patients (8).
Understanding the economic variations in the management of patients with MV could lead to effective interventions to improve care. However, little information is available about the economic variations in management and outcomes of patients with MV in different provinces of China. Considering that China’s economy and medical development are extremely uneven, the hypothesis of the present study is that economic differences between different provinces in China will lead to differences in the management of MV and in patient outcomes. Therefore, we conducted a national observational study to investigate the effect of economic variations on management and outcomes in mechanically ventilated patients in China.
Methods
Study design
We report post hoc analysis data describing the economic variations of patients with invasive MV from a multicentre observational cohort study in China. The study was a 1-month (August 31, 2012, to September 30, 2012) prospective observational study to describe the implementation of MV and the outcomes of mechanically ventilated patients in Chinese intensive care units (ICUs). The 1-month period was arbitrarily decided upon by the researchers to enroll more than 400 patients in the 20 participating ICUs. All 20 participating ICUs were closed ICUs in tertiary teaching hospitals in metropolitan cities, managed by full-time ICU doctors. Participating ICUs were selected according to convenience. The protocol was approved by the Institutional Ethics Committee of Zhongda Hospital (the core centre, Approval No. 2012ZD11KY09.0). Informed consent was waived due to the observational nature of the study. The trial was registered at clinicaltrials.gov (NCT01666834).
Study population
All patients who were admitted to participating ICUs during the study period were screened for eligibility. During the enrolment period, patients who received invasive MV for more than 24 hours were enrolled. We excluded those patients who were younger than 18 years old and who have been invasively ventilated prior to the study.
Data collection and definitions
Our data surveillance panel was organized by clinical experts to monitor every patient who was admitted to the participating ICUs to ensure that all patients with MV for more than 24 hours were sequentially included in our study. After the researchers had completed the case report form (CRF), an independent monitoring team screened the data of every patient who was admitted to the participating ICU during the study period to avoid missing any patient with MV.
For every enrolled patient, patient characteristics, underlying diseases, severity of illness, causes of MV, weaning process, and patient outcome were recorded. Ventilation settings, breathing patterns, sedative and analgesic were recorded on the first day of MV. Acute Physiology and Chronic Health Evaluation (APACHE) II score was assessed based on the worst variables recorded during the first 24 hours of ICU admission (9). All data were recorded at 9 a.m. each day, and the mean value of respiratory parameters in one minute was recorded. During assisted-controlled ventilation, plateau pressure and driving pressure analyses were confined to patients (n=448) in whom no evidence of spontaneous ventilation was detected (set and measured respiratory rates were equal). Plateau pressure was measured as airway pressure when the inspiratory flow rate was zero. Driving pressure was defined as plateau pressure minus positive end-expiratory pressure (PEEP). Protective ventilation was defined as tidal volume ≤8 mL/kg of predicted body weight (PBW) and plateau pressure ≤30 cmH2O. Ventilator-free days within 14 and 28 days after enrolment were recorded. If patients died during the 14- or 28-day period after enrolment, the number of ventilator-free days within 14 and 28 days was zero. All-cause ICU mortality, hospital mortality, 28-day mortality, ICU length of stay and hospital length of stay were recorded.
We used the gross domestic product (GDP) per capita in the province where each ICU is located to define high-income and middle-income groups. According to the 2012 World Bank countries classification of high-income countries, patients in the province with a GDP per capita over 12,615 US dollars per year were placed into the high-income group, while those with a GDP per capita between 1,046 to 12,615 US dollars per year were placed into the middle-income group.
Weaning classification was defined as follows: no weaning: comprising patients who never experienced any separation attempt. Short weaning: the first attempt resulted in a termination of the weaning process within 1 day (successful separation or early death). Difficult weaning: the weaning was completed after more than 1 day but in less than one week after the first separation attempt (successful weaning or death). Prolonged weaning: weaning was still not terminated 7 days after the first separation attempt (by success or death) (10). Separation attempt from MV: a spontaneous breathing trial (SBT) with or without extubation, or an extubation directly performed without identified SBT (whatever the type: planned or unplanned extubation) (10). Successful weaning: extubation without death or reintubation within the next 7 days, whichever came first.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 and STATA 14.1. Data are presented as the median (interquartile range). Continuous variables were compared with the use of one-way ANOVA or the Mann-Whitney test. Comparisons of proportions were made using Pearson’s chi-squared or Fisher’s exact tests. Nonparametric fitting was used to show the association between hospital mortality and tidal volume, peak pressure, plateau pressure and driving pressure graphically. Multivariate logistic regression analysis was used to detect the risk factors for hospital survival. Factors found to be significantly different between groups in univariate analysis were included in multivariate logistic regression analysis. Missing data were ignored during analysis. A significant difference was defined as P<0.05.
Results
Participating ICUs and patient characteristics
The doctor/bed ratio and nurse/bed ratio were comparable between ICUs from high-income and middle-income provinces (Table S1). Of these 483 participants, 291 (60.2%) were recruited from high-income provinces and 192 (39.8%) from middle-income provinces (Figure 1). Age, APACHE II score and comorbidities were not different between the high-income and middle-income groups (Table S2). As the causes of MV, ARDS was more common in the high-income group than in the middle-income group; however, coma was more common in the middle-income group than in the high-income group (Table 1).
Table S1. Characteristics of participating intensive care unites.
| Characteristic | All ICUs (n=20) | ICUs from high-income province (n=12) | ICUs from middle-income province (n=8) | P value |
|---|---|---|---|---|
| University affiliated hospital | 16 (80.0) | 9 (75.0) | 7 (87.5) | 0.494 |
| General ICU, n (%) | 19 (95.0) | 11 (91.7) | 8 (100.0) | 0.402 |
| Surgical ICU, n (%) | 1 (5.0) | 1 (8.3) | 0 (0) | 0.402 |
| Number of ICU beds, n | 25 [18–36] | 23 [16–36] | 25 [18–38] | 0.412 |
| Accounting for all hospital beds, % | 1.6 (1.1–2.1) | 1.7 (1.3–2.4) | 1.1 (0.7–1.8) | 0.990 |
| Doctor/bed ratio | 0.7 (0.5–0.8) | 0.7 (0.6–0.8) | 0.6 (0.5–0.8) | 0.244 |
| Nurse/bed ratio | 2.0 (2.0–2.5) | 2.0 (2.0–2.5) | 2.1 (1.7–2.5) | 0.793 |
Data are median (IQR) or number (%). ICU, intensive care units.
Figure 1.
Flow chart of patient screening and enrolment.
Table S2. Comorbidities of patients with invasive mechanical ventilation.
| Comorbidities | All (n=483) | High-income (n=291) | Middle-income (n=192) | P value |
|---|---|---|---|---|
| Respiratory system | ||||
| Smoke | 122 (25.3) | 70 (24.1) | 54 (28.1) | 0.339 |
| COPD | 26 (5.4) | 16 (5.5) | 10 (5.2) | 1.000 |
| Asthma | 8 (1.7) | 5 (1.7) | 3 (1.6) | 1.000 |
| Tumor | 9 (1.9) | 6 (2.1) | 3 (1.6) | 0.748 |
| Pulmonary tuberculosis | 22 (4.6) | 14 (4.8) | 8 (4.2) | 0.826 |
| Cardiovascular system | ||||
| Coronary artery disease | 90 (18.6) | 58 (19.9) | 32 (16.7) | 0.367 |
| Hypertension | 193 (40.0) | 112 (38.5) | 81 (42.2) | 0.417 |
| Chronic cardiac dysfunction | 37 (7.7) | 21 (7.2) | 16 (8.3) | 0.644 |
| History of PCI | 26 (5.4) | 16 (5.5) | 10 (5.2) | 0.901 |
| History of cardiosurgery | 8 (1.7) | 5 (1.7) | 3 (1.6) | 1000 |
| Diabetes | 104 (21.5) | 64 (22.0) | 40 (20.8) | 0.762 |
| Urinary system | ||||
| Chronic kidney dysfunction | 29 (6.0) | 18 (6.2) | 12 (6.3) | 0.977 |
| Hemodialysis | 13 (2.7) | 8 (2.7) | 5 (2.6) | 0.931 |
| Nervous system | ||||
| Stroke | 73 (15.1) | 43 (14.8) | 30 (15.6) | 0.799 |
| lying in bed ≥3 months | 23 (4.8) | 14 (4.8) | 9 (4.7) | 0.950 |
| Digestive system | ||||
| Cirrhosis | 8 (1.7) | 4 (1.4) | 4 (2.1) | 0.719 |
| Intestinal obstruction | 18 (3.7) | 10 (3.4) | 8 (4.2) | 0.678 |
| Immune system dysfunction | 20 (4.1) | 13 (4.5) | 7 (3.6) | 0.657 |
Data are number (%). APACHE II, Acute Physiology and Chronic Health Evaluation II; COPD, chronic obstructive pulmonary disease; PCI, percutaneous coronary intervention.
Table 1. Baseline characteristics and causes of mechanical ventilation.
| Variables | All (n=483) | High-income (n=291) | Middle-income (n=192) | P value |
|---|---|---|---|---|
| Age (years) | 64.0 (51.0–76.0) | 63.0 (50.0–76.0) | 64.0 (54.2–75.0) | 0.550 |
| Sex (male) | 341 (70.6) | 206 (70.8) | 135 (70.3) | 0.910 |
| High (cm) | 170.0 (162.0–173.0) | 170.0 (163.0–173.0) | 168.0 (162.0–173.0) | 0.458 |
| Real body weight (kg) | 65.0 (60.0–71.5) | 66.0 (6.0–72.0) | 65.0 (60.0–72.0) | 0.117 |
| Predicted body weight (kg) | 66.0 (56.9–68.8) | 66.0 (56.9–68.8) | 64.2 (54.2–68.5) | 0.545 |
| APACHE II | 19.0 (11.0–25.0) | 19.0 (11.0–24.0) | 19.0 (11.0–26.0) | 0.674 |
| Causes of mechanical ventilation | ||||
| Postoperative | 150 (31.1) | 89 (30.6) | 61 (31.8) | 0.783 |
| ARDS | 135 (28.0) | 92 (31.6) | 43 (22.4) | 0.030 |
| Coma | 82 (17.0) | 38 (13.1) | 44 (22.9) | 0.005 |
| Sever sepsis/septic shock | 33 (6.8) | 20 (6.9) | 13 (6.8) | 0.965 |
| Pneumonia | 27 (5.6) | 17 (5.8) | 10 (5.2) | 0.689 |
| AECOPD | 25 (5.2) | 16 (5.5) | 9 (4.7) | 0.694 |
| Congestive heart failure | 20 (4.1) | 15 (5.2) | 5 (2.6) | 0.243 |
| Neuromuscular diseases | 2 (0.4) | 1 (0.3) | 1 (0.5) | 0.096 |
| Others | 9 (1.9) | 3 (1.0) | 6 (3.1) | 0.767 |
Data are median (IQR) or number (%). APACHE II, Acute Physiology and Chronic Health Evaluation II; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; IQR, interquartile range.
MV
Synchronized intermittent mandatory ventilation + pressure support ventilation (SIMV + PSV) was the most popular mode used on the first day (385/483, 79.7%). Tidal volume, peak pressure, plateau and driving pressure were significantly lower in the high-income group than in the middle-income group (Table 2). The proportion of patients receiving protective ventilation (defined as a tidal volume ≤8 mL/kg of predicted bodyweight and plateau pressure of ≤30 cmH2O) was higher in the high-income group (71.1%) than in the middle-income group (59.9%) (P=0.014) (Table 2). After adjusting ARDS and coma at baseline, patients in the high-economic district were more likely to receive lung-protective ventilation [odds ratio (OR) 2.28; 95% CI: 1.51–3.41, P<0.001].
Table 2. Management of mechanical ventilation of patients with invasive mechanical ventilation.
| Variables | All (n=483) | High-income (n=291) | Middle-income (n=192) | P value |
|---|---|---|---|---|
| Ventilation mode | ||||
| SIMV + PS | 385 (79.7) | 235 (80.8) | 150 (78.1) | 0.482 |
| VCV | 7 (1.4) | 4 (1.4) | 3 (1.6) | 1.000 |
| PCV | 24 (5.0) | 20 (6.9) | 4 (2.1) | 0.018 |
| BIPAP | 28 (5.8) | 7 (2.4) | 21 (10.9) | 0.000 |
| PSV | 12 (2.5) | 10 (3.4) | 2 (1.0) | 0.136 |
| Other modes | 29 (6.0) | 18 (6.2) | 11 (5.7) | 0.836 |
| Recruitment maneuver | 58 (12.0) | 30 (10.3) | 28 (14.5) | 0.157 |
| Breathing patterns | ||||
| PEEP (cmH2O) | 5.0 (5.0–6.0) | 5.0 (5.0–7.0) | 5.0 (5.0–6.0) | 0.543 |
| FiO2 | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.4 (0.4–0.5) | 0.154 |
| Tidal volume (mL/kg PBW) | 7.4 (6.7–8.5) | 7.3 (6.7–8.3) | 7.7 (6.7–8.9) | 0.016 |
| RR (breath/min) | 17.0 (15.0–20.0) | 17.0 (15.0–20.0) | 18.0 (15.0–20.0) | 0.857 |
| Ti (second) | 1.0 (1.0–1.2) | 1.0 (1.0–1.2) | 1.0 (1.0–1.3) | 0.948 |
| Peak pressure (cmH2O) | 21.0 (18.0–25.0) | 20.0 (18.0–25.0) | 22.0 (19.0–28.0) | 0.000 |
| Plateau pressure (cmH2O) | 17.0 (15.0–20.0) | 16.0 (15.0–19.0) | 18.0 (15.0–20.0) | 0.041 |
| Driving pressure (cmH2O) | 11.0 (8.0–14.0) | 11.0 (8.0–13.0) | 12.0 (9.0–15.0) | 0.045 |
| Receiving protective ventilationa | 314 (65.0) | 207 (71.1) | 115 (59.9) | 0.014 |
| Sedative | 368 (76.2) | 219 (75.3) | 149 (77.6) | 0.553 |
| Analgesic | 289 (59.8) | 175 (60.1) | 114 (59.4) | 0.867 |
| Both analgesic and sedative | 267 (55.3) | 158 (54.3) | 109 (56.8) | 0.592 |
| Daily interruption of sedation | 276 (57.1) | 188 (64.6) | 88 (45.8) | <0.001 |
| Extubation with SBT | 290 (60.0) | 184 (63.2) | 106 (55.2) | 0.078 |
| T piece | 142 (29.4) | 98 (33.7) | 44 (22.9) | 0.011 |
| PSV | 124 (25.7) | 72 (24.7) | 52 (27.1) | 0.564 |
| CPAP | 24 (5.0) | 14 (4.8) | 10 (5.2) | 0.844 |
Data are median (IQR) or number (%). a, tidal volume ≤8 mL/kg of predicted bodyweight and plateau pressure ≤30 cmH2O. SIMV + PS, synchronized intermittent mandatory ventilation + pressure support ventilation; VCV, volume control ventilation; PCV, pressure support ventilation; BIPAP, bi-level airway pressure ventilation; PSV, pressure support ventilation; PEEP, positive end expiratory pressure; FiO2, fraction of inspired oxygen; PBW, predicted body weight; RR, respiratory rate; Ti, inspiratory time; SBT, spontaneous breathing trial; NIV, non-invasive mechanical ventilation; SBT, spontaneous breathing trial; IQR, interquartile range.
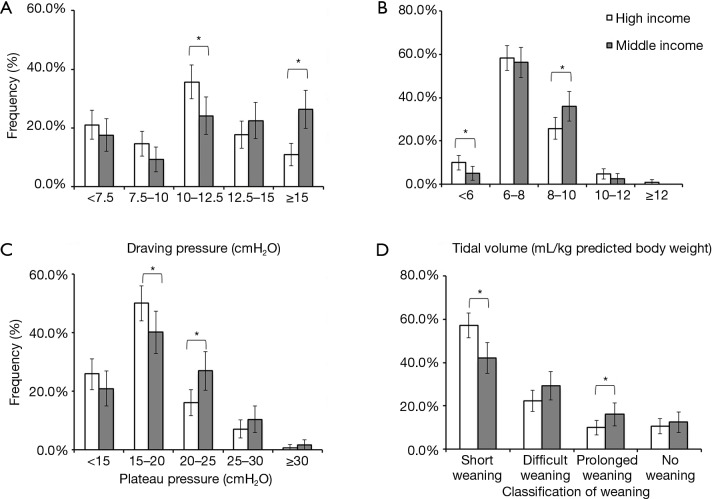
More patients received lower driving pressure (<15 cmH2O) and tidal volume (<6 mL/kg of PBW) in the high-income group than in the middle-income group (Figure 2A,B). As shown in Figure 2C, more patients in the high-income group distributed more in the low plateau pressure (P<0.05). In all mechanically ventilated patients, with the increase of peak pressure, plateau pressure and driving pressure, hospital mortality gradually increased. However, the tidal volume within the 6–8 mL/kg of PBW was associated with the lowest hospital mortality (Figure S1).
Figure 2.
Driving pressure (A), tidal volume (B), plateau pressure (C), and classification of weaning (D), by income. Error bars represent 95% CIs; *, P<0.05. No weaning: comprising patients who never experienced any separation attempt. Short weaning: the first attempt resulted in a termination of the weaning process within 1 day (successful separation or early death). Difficult weaning: the weaning was completed after more than 1 day but less than 1 week after the first separation attempt (successful weaning or death). Prolonged weaning: weaning was not terminated 7 days after the first separation attempt (by success or death).
Figure S1.
Graphically depiction of the association between hospital mortality and tidal volume (A), peak airway pressure (B), airway platform pressure (C), and driving pressure (D).
Analgesia and sedation
The proportion of patients receiving analgesic, sedative, or both did not differ significantly between groups (Table 2). The ratio of midazolam application was much higher in the middle-income group than in the high-income group (Table S3). Daily interruption of sedatives was significantly more common in the high-income group than in the middle-income group (Table 2).
Table S3. Sedative and analgesic in patients with invasive mechanical ventilation.
| Sedative and analgesic | All (n=483) | High-income (n=291) | Middle-income (n=192) | P value |
|---|---|---|---|---|
| Propofol | 225 (46.6) | 138 (47.4) | 87 (45.3) | 0.649 |
| Midazolam | 94 (19.5) | 46 (15.8) | 48 (25.0) | 0.013 |
| Diazepam | 3 (0.6) | 2 (0.7) | 1 (0.5) | 1.000 |
| Dexmedetomidine | 121 (25.1) | 72 (24.7) | 49 (25.5) | 0.847 |
| Morphine | 142 (29.4) | 82 (28.2) | 60 (31.3) | 0.468 |
| Fentanyl | 81 (16.8) | 49 (16.8) | 32 (16.7) | 0.961 |
| Remifentanil | 57 (11.8) | 41 (14.1) | 16 (8.3) | 0.055 |
| Sufentanil | 1 (0.2) | 1 (0.3) | 0 (0) | 1.000 |
Liberation from MV
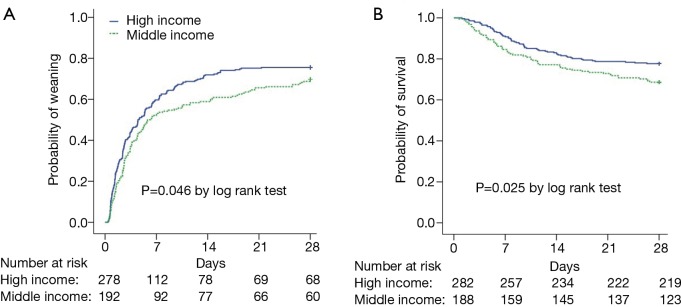
Short weaning was more common and prolonged weaning was less common in the high-income group than in the middle-income group (P<0.05, Figure 2D). Kaplan-Meier analyses showed that the probability of weaning was greater in the high-income group than in the middle-income group (P=0.046, Figure 3A). Compared with patients in the middle-income group, those in the high-income group had significantly higher median numbers of ventilator-free days within 14 and 28 days (Table 3). Multivariate logistic regression showed that SBT was an independent protection factor associated with hospital mortality (Table 3).
Figure 3.
Kaplan-Meier curves of probability of weaning from mechanical ventilation (A) and hospital survival (B), by income. Patients discharged before day 28 were assumed to be alive and off invasive mechanical ventilation on that day.
Table 3. Outcomes of patients with invasive mechanical ventilation.
| Outcomes | All (n=483) | High-income (n=291) | Middle-income (n=192) | P value |
|---|---|---|---|---|
| Successful weaning | 366 (75.6) | 223 (76.6) | 143 (74.5) | 0.589 |
| Classification of weaning | ||||
| Short weaning | 247 (51.1) | 166 (57.0) | 81 (42.2) | 0.001 |
| Difficult weaning | 121 (25.1) | 65 (22.3) | 56 (29.2) | 0.107 |
| Prolonged weaning | 60 (12.4) | 29 (10.0) | 31 (16.1) | 0.044 |
| No weaning | 55 (11.4) | 31 (10.7) | 24 (12.5) | 0.664 |
| Preventative NIV after extubation | 33 (6.8) | 16 (5.5) | 17 (8.9) | 0.153 |
| Tracheostomy | 45 (9.3) | 25 (8.6) | 20 (10.4) | 0.499 |
| Ventilator-free days, day 14 (days) | 8.6 (0–12.1) | 8.8 (0–12.3) | 8.0 (0–11.6) | 0.046 |
| Ventilator-free days, day 28 (days) | 22.3 (0–26.1) | 22.6 (0–26.2) | 21.2 (0–25.6) | 0.020 |
| Length of stay in ICU (days) | 22.3 (0–26.1) | 5.8 (2.5–10.6) | 5.7 (2.5–11.4) | 0.791 |
| Length of stay in hospital (days) | 16.3 (8.2–28.4) | 16.4 (9.2–26.9) | 15.4 (6.7–29.8) | 0.821 |
| ICU mortality | 118 (24.4) | 70 (24.1) | 48 (25.0) | 0.813 |
| 28-day mortality | 123 (25.5) | 64 (22.0) | 59 (30.7) | 0.031 |
| Hospital mortality | 152 (31.5) | 83 (28.5) | 69 (35.9) | 0.086 |
Data are median (IQR) or number (%). No weaning: comprising patients who never experienced any separation attempt. Short weaning: the first attempt resulted in a termination of the weaning process within one day (successful separation or early death). Difficult weaning: the weaning was completed after more than one day but in less than one week after the first separation attempt (successful weaning or death). Prolonged weaning: weaning was still not terminated 7 days after the first separation attempt (by success or death). ICU, intensive care unit; IQR, interquartile range; NIV, non-invasive mechanical ventilation.
Outcomes
Twenty-eight-day survival was higher in the high-income group than in the middle-income group (P=0.025, Figure 3B). However, hospital mortality did not differ between the high-income group (28.5%) and the middle-income group (35.9%, P=0.086). Length of stay in the ICU and in the hospital were comparable between the two groups (Table 3). Univariate analysis showed that patient-level variables associated with hospital mortality included age, SBT, daily interruption of sedation, the first day of APACHE II, PEEP, tidal volume, plateau pressure, peak pressure, driving pressure, lactic acid, and partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2). PEEP was independently associated with in-hospital mortality. PEEP was also correlated with PaO2/FiO2 (r=−0.309, 95% CI: −0.380 to −0.232, P<0.001) and APACHE II score (r=0.133, 95% CI: 0.051–0.219, P=0.003). Driving pressure, PEEP and SBT were independently associated with in-hospital mortality (Table 4).
Table 4. Factors associated with hospital mortality (n=448).
| Factors | Univariate analysis | Multivariate logistic regression analysis | |||
|---|---|---|---|---|---|
| Odds ratio (95% confidence interval) | P value | Odds ratio (95% confidence interval) | P value | ||
| Genderb | 0.751 (0.487–1.159) | 0.196 | 0.911 (0.497–1.667) | 0.076 | |
| Ageb | 1.014 (1.002–1.027) | 0.016 | 1.013 (0.998–1.030) | 0.092 | |
| The first day of APPACHE IIa | 1.094 (1.067–1.122) | <0.001 | 1.029 (0.996–1.065) | 0.087 | |
| Daily interruption of sedationa | 0.430 (0.285–0.642) | <0.001 | 0.715 (0.403–1.271) | 0.254 | |
| PEEP (cmH2O)a | 1.186 (1.086–1.294) | <0.001 | 1.280 (1.132–1.447) | <0.001b | |
| Tidal volume (mL/kg PBW)a | |||||
| <6 | 2.827 (1.395–5.726) | 0.004 | 1.957 (0.795–4.814) | 0.144 | |
| 6–8 | 1.000 (reference) | – | 1.000 (reference) | – | |
| 8–10 | 1.700 (1.080–2.676) | 0.022 | 1.433 (0.788–2.606) | 0.238 | |
| >10 | 2.424 (1.104–5.322) | 0.027 | 2.276 (0.747–6.937) | 0.148 | |
| Plateau pressure (cmH2O) | 1.123 (1.073–1.176) | <0.001 | – | – | |
| Peak pressure (cmH2O) | 1.083 (1.042–1.127) | <0.001 | – | – | |
| Driving pressure (cmH2O)a | 1.065 (1.021–1.111) | 0.003 | 1.084 (1.019–1.153) | 0.010 b | |
| Blood gas | |||||
| pH | 0.902 (0.484–1.681) | 0.745 | – | – | |
| HCO3−a | 0.960 (0.924–0.997) | 0.034 | 0.977 (0.938–1.019) | 0.285 | |
| Laca | 1.093 (1.016–1.176) | 0.017 | 1.045 (0.989–1.105) | 0.115 | |
| PaO2 | 0.996 (0.992–1.000) | 0.077 | – | – | |
| PaCO2 | 0.996 (0.976–1.017) | 0.714 | – | – | |
| PaO2/FiO2a | 0.997 (0.996–0.999) | 0.002 | 1.001 (0.999–1.004) | 0.278 | |
| SBTa | 0.086 (0.053–0.138) | <0.001 | 0.118 (0.067–0.206) | <0.001b | |
a, the variables placed in the multivariate logistic regression model; b, the significant variables selected by the stepwised method in the multivariate logistic regression. APACHE II, Acute Physiology and Chronic Health Evaluation II; PEEP, positive end expiratory pressure; PBW, predicted body weight; SBT, spontaneous breathing trial; HCO3−, bicarbonate; Lac, lactic acid.
Discussion
In this prospective cross-sectional study, we noted important differences in the management of MV and patient outcomes according to economic variation in China. Patients from high-income provinces had lower tidal volume, plateau and driving pressures; a higher proportion of receiving protective ventilation; shorter weaning; and better survival in the 28 days. This information will help to improve the management of MV, especially in middle-income provinces.
Patient characteristics and outcomes
We found a somewhat lower proportion of mechanically ventilated patients in ICUs and hospital mortality in this study than in the global data. A system analysis of 18,302 patients from 927 ICUs showed a ventilation rate of 35% in ICU patients and a hospital mortality of 35% in 2010 (3). In the present study, 26.6% of patients received invasive MV and the hospital mortality was 28.5%. However, another survey by Du et al. showed a high invasive ventilation rate (68.8%) in patients admitted to 22 Chinese ICUs (11). The most likely explanation is the difference in enrolment criteria and participating ICUs between the two surveys. However, the initial APACHE II scores were comparable (19 vs. 18), which suggests that severity of illness might not be the main reason for the difference between the two Chinese surveys. We found a similar constituent ratio of the main reason for MV between this study and others (1-3), and postoperative respiratory support was the most common reason for invasive MV.
Although patients from high-income and middle-income provinces did not differ in terms of demographics, survival within the 28 days was significantly higher in the high-income group than in the middle-income group. Given the comparable ratio of doctors/beds and nurses/beds between the two groups, the shortage of human resources might not be the main reason of the worse survival rate among patients from middle-income provinces when compared with those from high-income provinces. Although in terms of causes of MV, ARDS was more common in the high-income group and coma was more common in the middle-income group, this difference still cannot be a reasonable explanation for the difference in survival between groups. Part of the potential explanation for that difference might include differences in practices of MV and weaning from ventilation between groups. Other potential explanations might be differences in continuation of further life support care, as the family might not be able to afford the high costs. This might be more prevalent in the middle-income group when compared with the high-income group. However, the present study did not record the withholding and withdrawing populations.
Management during MV
High-income provinces had a higher proportion of patients receiving protective ventilation (defined as a tidal volume ≤8 mL/kg of PBW and plateau pressure of ≤30 cmH2O) than middle-income provinces. This might be one of the potential explanations for the better survival of patients from high-income provinces. Tidal volume was the essential component of lung-protective ventilatory strategies, and low tidal volume may be important in patients with ARDS (12). However, in patients without ARDS, the benefits of low tidal volume remain controversial (13-15). We found that when compared with a tidal volume of 6–8 mL/kg, a higher tidal volume was associated with a worse outcome. The higher tidal volume in the middle-income group than in the high-income group was a potential explanation of the lower survival in the middle-income group.
Higher driving pressure has been associated with poor outcomes in ARDS patients (16). However, driving pressure, which is an attractive method of setting tidal volume, requires normalization to the patient’s respiratory system compliance and was questioned in the cohort of patients without ARDS. Within the limits of the ventilatory setting normally used by clinicians, driving pressure was not associated with hospital mortality (17). In a heterogeneous population or cohort of patients with MV (28.0% patients with ARDS), we found an association between driving pressure and hospital mortality. Our data supported the idea of altering the mechanical scenario to decrease the driving pressure even in heterogeneous patients (18).
Since the main cause of MV was postoperative respiratory support in the present study, it was no surprise that patients received uniform physiological levels of PEEP in both groups. PEEP is an essential component of lung-protective ventilatory strategies not only in ARDS patients but also in other patients with MV. Observational studies have reported an independent association between zero PEEP and mortality in a heterogeneous cohort of mechanically ventilated patients (19). In the present study, we found that PEEP was an independent risk factor for hospital mortality. Although high PEEP may cause harm if not necessary, the negative correlation between PEEP and PaO2/FiO2 and the positive correlation between PEEP and the APACHE II score in the present study might suggest that patients with a severe disease and poor oxygenation are more likely to receive higher PEEP.
Analgesia, sedation and liberation from MV
Patients from high-income provinces had higher numbers of ventilator-free days within 14 and 28 days and a higher probability of weaning within 28 days than those from middle-income provinces. SBT has been considered an essential step for the decision to extubate (20-23). We found an independent association between SBT and hospital mortality, which suggests that differences in weaning practice partly explained the effect of income on weaning duration. The implementation of sedative and analgesia largely affects weaning duration (24-26). Daily interruption of sedation might relieve continuous deep sedation and decrease the duration of MV (27). The potential explanations of the more common short weaning in patients from high-income provinces include the higher proportion of daily interruption of sedatives than those in patients from middle-income provinces. Other potential explanations included the lower frequency of midazolam used in the high-income group than in the middle-income group. Short weaning has been associated with better outcomes (10); this was another potential explanation of the higher survival rate within the 28 days in patients from high-income provinces. We also found that the proportion of prolonged weaning was higher in patients from the middle-income group; however, the proportion of successful weaning was comparable between groups. These findings implied the delayed weaning in patients from middle-income provinces.
With the exception of 55 (11.4%) patients who never experienced any separation attempt, we classified patients into short, difficult and prolonged weaning based on weaning duration according to the WIND study (10). Our data suggest that the three groups have different weaning outcomes and mortality. Consistent with previous reports, we found that most patients were in the short weaning group, and patients in the prolonged group had the worst survival in 28 days.
Analgesia and sedation are important components of care for the invasive mechanically ventilated patients in the ICU. It is vital to remember that protocols attempting to minimize sedation must first acknowledge the need for adequate pain control (28-30). Consistent with other studies (31,32), we found that only 59.8% of patients with MV received analgesic (no difference between high-income and middle-income groups). Measures to improve the insufficient application of analgesic should be taken in ICUs in China.
Limitations
Our study has several limitations. First, our cohort is a convenience sample from 20 participating ICUs from nine provinces and therefore might not be representative of actual clinical practice in ICUs in China. Thus, our results could be biased, particularly where specific types of ICUs (e.g., academic ICUs) might be over-represented. Second, the research period (summer) was arbitrarily chosen, which could introduce bias when extrapolating the results to a whole year. However, another study showed that age, severity, the proportion of MV and mortality were not subject to seasonal effects (33). Third, because we only recorded the ventilator parameters during the early phase of MV, the present study was unable to show the graph of the entire MV process and comparison between groups. Fourth, it might not be appropriate to classify groups according to the high and middle incomes of each province in one county according to the 2012 World Bank country classification by GDP per capita. Fifth, inspiration occlusions were not used during the measurement of plateau pressure and driving pressure, and thus the absolute value of the plateau pressure and driving pressure might be slightly overestimated.
Conclusions
This prospective cross-sectional study showed important economic differences in the practice of MV and weaning and in patient outcomes in China. Higher income was associated with a higher proportion of protective ventilation, shorter weaning and better survival in patients with MV. These findings may serve as a current benchmark of the usual care and outcomes of patients with MV in provinces with different income levels in China. They may also indicate a potential for improvement in patient management.
Acknowledgments
We are indebted to Mrs. Xiaojin Yu and Mr. Zi Xiang for assisting with data analysis. We thank all the members of the present study for their helpful and continuous support, including Dan Ao (Lishui People’s Hospital), Wenkui Yu (Nanjing General Hospital of Nanjing Military Command), Yushan Wang (The First Hospital of Jilin University), Kang Chen (Zhangjiagang First People’s Hospital), Jie Yan (Wuxi People’s Hospital), Jianguo Li (Zhongnan Hospital, Wuhan University), Guolong Cai (Zhejiang Hospital), Yurong Wang (Yangzhou First People’s Hospital), Hongliang Wang (The Second Affiliated Hospital, Harbin Medical University), and Yan Kang (West China School of Medicine).
Funding: This study was supported by The Ministry of Health of China (Special Fund for Health-Scientific Research in the Public Interest Program 201202011), the National Natural Science Foundation of China (81670074, 81870066), the Natural Science Foundation of Jiangsu Province (BK20171271), Jiangsu Provincial Medical Youth Talent (QNRC 2016807), and Third Level Talents of the “333 High Level Talents Training Project” in the fifth phase in Jiangsu (LGY2016051).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The protocol was approved by the Institutional Ethics Committee of Zhongda Hospital (the core centre, Approval No. 2012ZD11KY09.0).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Esteban A, Anzueto A, Frutos F, et al. Mechanical ventilation international study group characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA 2002;287:345-55. 10.1001/jama.287.3.345 [DOI] [PubMed] [Google Scholar]
- 2.Esteban A, Ferguson ND, Meade MO, et al. Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 2008;177:170-7. 10.1164/rccm.200706-893OC [DOI] [PubMed] [Google Scholar]
- 3.Esteban A, Frutos-Vivar F, Muriel A, et al. Evolution of mortality over time in patients receiving mechanical ventilation. Am J Respir Crit Care Med 2013;188:220-30. 10.1164/rccm.201212-2169OC [DOI] [PubMed] [Google Scholar]
- 4.Likosky DS, Al-Attar PM, Malenka DJ, et al. Geographic variability in potentially discretionary red blood cell transfusions after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg 2014;148:3084-9. 10.1016/j.jtcvs.2014.07.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones WS, Patel MR, Dai D, et al. Temporal trends and geographic variation of lower-extremity amputation in patients with peripheral artery disease: results from US Medicare 2000–2008. J Am Coll Cardiol 2012;60:2230-6. 10.1016/j.jacc.2012.08.983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raghupathy A, Nienaber CA, Harris KM, et al. Geographic differences in clinical presentation, treatment, and outcomes in type A acute aortic dissection (from the International Registry of Acute Aortic Dissection). Am J Cardiol 2008;102:1562-6. 10.1016/j.amjcard.2008.07.049 [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Greiner MA, DiMartino LD, et al. Geographic variation in carotid revascularization among Medicare beneficiaries, 2003–2006. Arch Intern Med 2010;170:1218-25. 10.1001/archinternmed.2010.194 [DOI] [PubMed] [Google Scholar]
- 8.Laffey JG, Madotto F, Bellani G, et al. Geo-economic variations in epidemiology, patterns of care, and outcomes in patients with acute respiratory distress syndrome: insights from the LUNG SAFE prospective cohort study. Lancet Respir Med 2017;5:627-38. 10.1016/S2213-2600(17)30213-8 [DOI] [PubMed] [Google Scholar]
- 9.Knaus WA, Draper EA, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 10.Béduneau G, Pham T, Schortgen F, et al. Weaning accordIng New Definition (WIND) study group on behalf of Réseau Européen de recherche en Ventilation Artificielle (REVA) network. Epidemiology of weaning outcome according to a new definition. The WIND Study. Am J Respir Crit Care Med 2017;195:772-83. [DOI] [PubMed] [Google Scholar]
- 11.Du B, An Y, Kang Y, et al. China Critical Care Clinical Trial Group Characteristics of critically ill patients in ICUs in mainland China. Crit Care Med 2013;41:84-92. 10.1097/CCM.0b013e31826a4082 [DOI] [PubMed] [Google Scholar]
- 12.Gajic O, Frutos-Vivar F, Esteban A, et al. Ventilator settings as a risk factor for acute respiratory distress syndrome in mechanically ventilated patients. Intensive Care Med 2005;31:922-6. 10.1007/s00134-005-2625-1 [DOI] [PubMed] [Google Scholar]
- 13.Determann RM, Royakkers A, Wolthuis EK, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care 2010;14:R1. 10.1186/cc8230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: A meta-analysis. JAMA 2012;308:1651-9. 10.1001/jama.2012.13730 [DOI] [PubMed] [Google Scholar]
- 15.Writing Group for the PReVENT Investigators , Simonis FD, Serpa Neto A, et al. Effect of a Low vs Intermediate Tidal Volume Strategy on Ventilator-Free Days in Intensive Care Unit Patients Without ARDS: A Randomized Clinical Trial. JAMA 2018;320:1872-80. 10.1001/jama.2018.14280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato MB, Meade MO, Slutsky AS, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747-55. 10.1056/NEJMsa1410639 [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MFS, Amaral ACKB, Fan E, et al. Driving pressure and hospital mortality in patients without ARDS: A cohort study. Chest 2018;153:46-54. 10.1016/j.chest.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 18.Borges JB, Hedenstierna G, Larsson A, et al. Altering the mechanical scenario to decrease the driving pressure. Crit Care 2015;19:342. 10.1186/s13054-015-1063-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metnitz PG, Metnitz B, Moreno RP, et al. SAPS 3 Investigators. Epidemiology of mechanical ventilation: analysis of the SAPS 3 database. Intensive Care Med 2009;35:816-25. 10.1007/s00134-009-1449-9 [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med 1996;335:1864-9. 10.1056/NEJM199612193352502 [DOI] [PubMed] [Google Scholar]
- 21.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet 2008;371:126-34. 10.1016/S0140-6736(08)60105-1 [DOI] [PubMed] [Google Scholar]
- 22.Zeggwagh AA, Abouqal R, Madani N, et al. Weaning from mechanical ventilation: a model for extubation. Intensive Care Med 1999;25:1077-83. 10.1007/s001340051015 [DOI] [PubMed] [Google Scholar]
- 23.Ouellette DR, Patel S, Girard TD, et al. Liberation from mechanical ventilation in critically ill adults: an official American college of Chest Physicians/American Thoracic Society clinical practice guideline inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest 2017;151:166-80. 10.1016/j.chest.2016.10.036 [DOI] [PubMed] [Google Scholar]
- 24.Stephens RJ, Ablordeppey E, Drewry AM, et al. Practices and the Impact of Sedation Depth on Clinical Outcomes Among Patients Requiring Mechanical Ventilation in the ED: A Cohort Study. Chest 2017;152:963-71. 10.1016/j.chest.2017.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shehabi Y, Chan L, Kadiman S, et al. Sedation depth and long-term mortality in mechanically ventilated critically ill adults: a prospective longitudinal multicentre cohort study. Intensive Care Med 2013;39:910-8. 10.1007/s00134-013-2830-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shehabi Y, Bellomo R, Reade MC, et al. Early intensive care sedation predicts long-term mortality in ventilated critically ill patients. Am J Respir Crit Care Med 2012;186:724-31. 10.1164/rccm.201203-0522OC [DOI] [PubMed] [Google Scholar]
- 27.Kress JP, Pohlman AS, O’Connor MF, et al. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med 2000;342:1471-7. 10.1056/NEJM200005183422002 [DOI] [PubMed] [Google Scholar]
- 28.Schmidt GA, Girard TD, Kress JP, et al. Official executive summary of an American Thoracic Society/American College of chest physicians clinical practice guideline: liberation from mechanical ventilation in critically Ill adults. Am J Respir Crit Care Med 2017;195:115-9. 10.1164/rccm.201610-2076ST [DOI] [PubMed] [Google Scholar]
- 29.Barr J, Fraser GL, Puntillo K, et al. American College of Critical Care Medicine. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263-306. 10.1097/CCM.0b013e3182783b72 [DOI] [PubMed] [Google Scholar]
- 30.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825-73. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 31.Haberthür C, Mols G, Elsasser S, et al. Extubation after breathing trials with automatic tube compensation, T-tube, or pressure support ventilation. Acta Anaesthesiol Scand 2002;46:973-9. 10.1034/j.1399-6576.2002.460808.x [DOI] [PubMed] [Google Scholar]
- 32.Esteban A, Alía I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T-tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med 1997;156:459-65. 10.1164/ajrccm.156.2.9610109 [DOI] [PubMed] [Google Scholar]
- 33.Santana Cabrera L, Sánchez-Palacios M, Uriarte Rodriguez A, et al. Seasonal influence in characteristics of patients admitted to an intensive care unit. Med Intensiva 2010;34:102-6. 10.1016/j.medin.2009.07.003 [DOI] [PubMed] [Google Scholar]