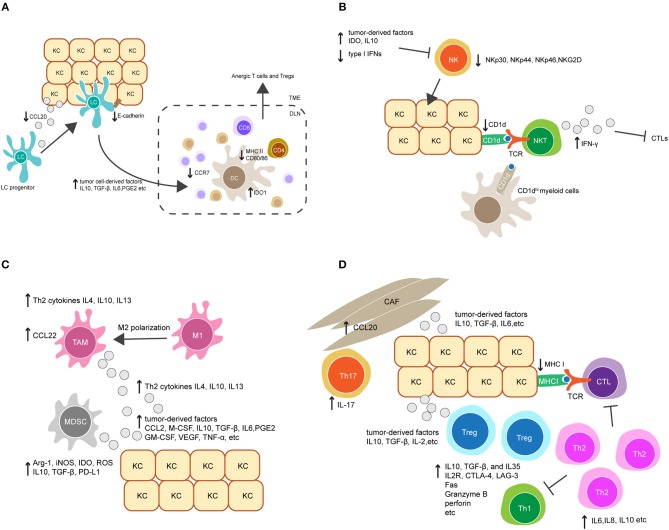
Figure 2.
Crosstalk between keratinocytes (KCs) and immune cells orchestrates immunosuppression in HPV-associated tumor microenvironment. (A) Downregulation of the chemokine CCL20 and cell adhesion molecule E-cadherin in HPV-infected KCs reduces Langerhans cells (LCs) infiltration in HPV-associated tumor. LCs and migratory dendritic cells (DCs) in tumor microenvironment also display immature or regulatory phenotypes and have reduced migratory capacity to secondary lymphoid tissue, characterized by downregulation of MHC II, CD80, CD86, and the chemokine receptor CCR7, and upregulation of indoleamine 2, 3-dioxygenase 1 (IDO1). This is probably mediated by tumor cell-derived immunosuppressive factors, such as IL-10, TGF-β, IL-6, and prostaglandin E2 (PGE2). (B) Upregulation of tumor-derived factors and downregulation of type I IFNs in HPV-associated tumor suppress NK cell activation and its killing capacity against tumor cells. On the other hand, downregulation of CD1d on KCs dampens NKT cell activity. CD1dhi myeloid cells in tumor environment might contribute to an alternative source of CD1d, leading to the activation of immunosuppressive IFN-γ-producing NKT cells. (C) The accumulation of tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) is mediated by a variety of tumor-derived factors, such as the chemokine CCL2, macrophage colony-stimulating factor (M-CSF), IL10, TGF-β, IL6 and PGE2. Additionally, Th2-associated cytokines promote TAM differentiation by inducing a phenotypic switch from M1 to M2. TAMs produce Th2-associated cytokines to promote Th2 cell differentiation, and secret the chemokine CCL22 to recruit Tregs. Similarly, MDSCs inhibit the effector immune response by producing a broad range of suppressive molecules, such as arginase 1 (Arg-1), inducible nitric oxide synthase (iNOS), IDO, reactive oxygen species (ROS), IL10, TGF-β, and PD-L1. (D) Tumor-derived factors promote the accumulation of Tregs and a shift from a Th1 toward a Th2 response in local microenvironment. In addition, the recruitment of Th17 cells is increased by CCL20 secretion in cancer-associated fibroblasts (CAFs). Collectively, these modulated responses might in part contribute to downregulation of CTL responses.