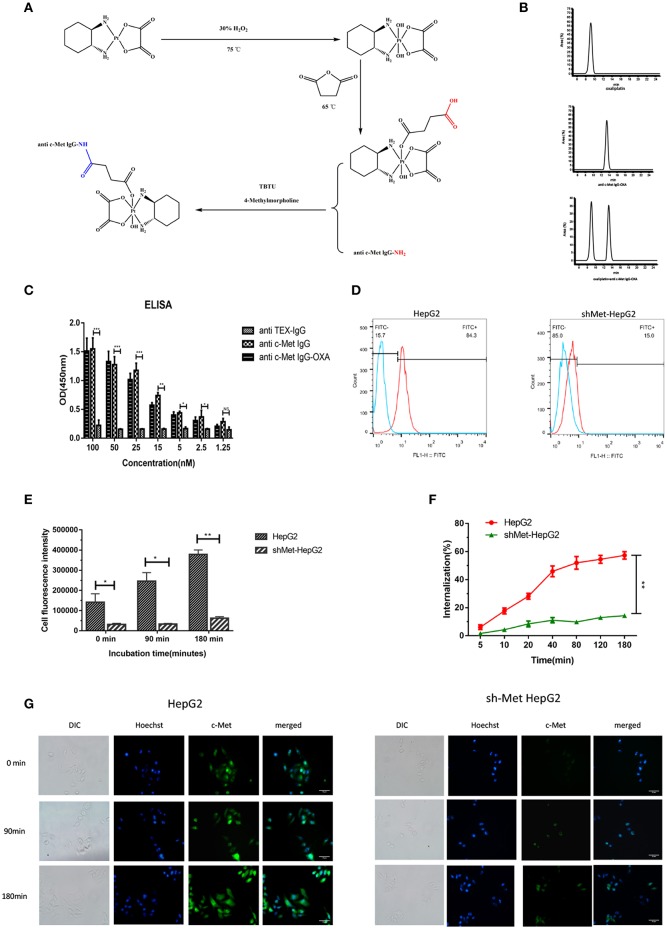
Figure 1.
Synthetic schemes and characterization of humanized antibody IgG against c-Met with oxaliplatin. (A) A monoclonal antibody (anti c-Met IgG) connected by a non-cleavable linker to oxaliplatin using EDC/NHS chemistry. (B) RP-HPLC was used to identify anti-c-Met IgG conjugated to oxaliplatin at an absorbance wavelength of 280 nm. (C,D) The conjugate binding examination of the affinity of anti-c-Met IgG-OXA by the enzyme-linked immunosorbent assay, incubated with various concentrations of anti-c-Met IgG and anti-c-Met IgG-OXA, using anti-TEX IgG as the isotype control antibody. The signal-to-background ratios of anti-c-Met antibody revealed strong antigen-specific responses to c-Met protein. Moreover, the ELISA results also showed that the conjugates had the same affinity reactivity to the recombinant protein of c-Met. *p < 0.05, **p < 0.01, and ***p < 0.001. NS: not significant. Flow cytometry analysis of the binding of the HepG2 cell lines and shMet-HepG2 to anti-c-Met IgG-OXA was measured using FCM. Flow cytometry histograms demonstrating that anti-c-Met IgG-OXA bound specifically to the cell surface protein of c-Met. (E–G) The ratio of anti-c-Met IgG-OXA endocytosis was measured by flow cytometry for 0–180 min. The data showed that anti-c-Met IgG-OXA was internalized into HepG2 cells rapidly within 48 min and stabilized at 90 min. Compared with c-Met positive cell lines, there was a significant difference in shMet-HepG2 cells treated with the conjugates. The bars of internalization at 180 min represent the mean ± SD. Data represent three experiments performed in triplicate. *P < 0.05 and **P < 0.01. Different degrees of binding and internalization of the conjugates were observed in HepG2 and shMet-HepG2 cells by immunofluorescence microscopy (200×). Scar bars represent 10 μm. The degree of internalization of the conjugates in HepG2 cells increased over time, while the shMet-HepG2 cells showed barely any internalization.