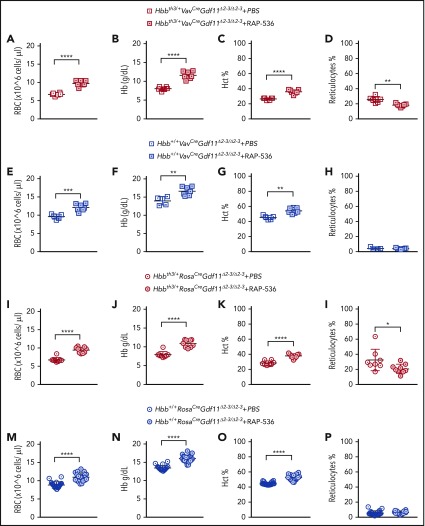
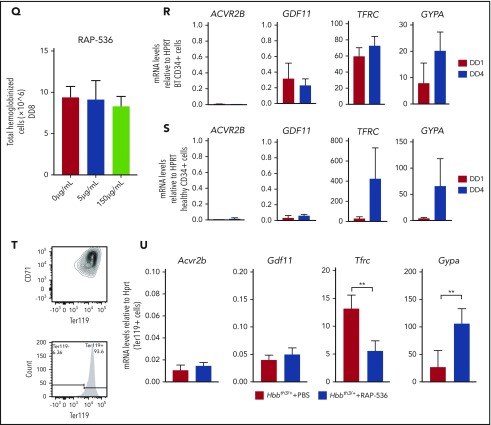
Figure 2.
Gdf11 deletion from the hematopoietic compartment or pancellularly from all tissues did not suspend RAP-536 action. Hbbth3/+VavCreGdf11Δ2-3/Δ2-3 mice treated with RAP-536 (n = 7) showed increased RBCs (A), Hb (B), and Hct (C), as well as reduced reticulocytes (D), compared with phosphate-buffered saline (PBS)-treated controls (n = 6). Hbb+/+VavCreGdf11Δ2-3/Δ2-3 mice also exhibited increased RBCs (E), Hb (F), and Hct (G). No statistically significant difference was found in reticulocyte number (H). VavCreGdf11Δ2-3/Δ2-3 mice were treated with RAP-536 between 3 and 4 months. Similarly, Hbbth3/+RosaCreGdf11Δ2-3/Δ2-3 mice (n = 9) and Hbb+/+RosaCre Gdf11Δ2-3/Δ2-3 mice (n = 20) treated with RAP-536 exhibited increased RBC (I,M), Hb (J,N), and Hct (K,O) levels compared with Hbbth3/+RosaCreGdf11Δ2-3/Δ2-3 (n = 8) and Hbb+/+RosaCre Gdf11Δ2-3/Δ2-3 (n = 20) PBS-treated animals. (L) Hbbth3/+RosaCreGdf11Δ2-3/Δ2-3 mice showed a significant reduction in reticulocytes. (P) No statistical differences were observed in reticulocytes from Hbb+/+RosaCre Gdf11Δ2-3/Δ2-3 animals. RosaCre Gdf11Δ2-3/Δ2-3 animals were treated with RAP-536 between 4 and 7 months. CBCs were assessed 2 days after last dose of RAP-536 treatment. Females and males were included in the analysis of all groups. CD34+ cells did not respond to RAP-536 in vitro. (Q) Treatment of CD34+-derived cells isolated from healthy donors with 5 µg/mL or 150 µg/mL RAP-536 (n = 3) did not result in increased cell number at the end of erythroid differentiation assay day 8 (DD8). mRNA levels of GDF11 and ACVR2B were low in human thalassemia and healthy erythroid progenitor cells. Quantification of GDF11 and ACVR2B mRNA in thalassemia (R) and healthy (S) donor-derived erythroblasts showed low GDF11 and ACVR2B expression relative to HPRT after day 1 (DD1) and day 4 (DD4) of differentiation in an erythroid differentiation assay, as determined by qRT-PCR (n = 3). BT CD34+ cells showed higher relative levels of GDF11 compared with healthy CD34+ cells. RHO was used as a negative control (data not shown), and TFRC and GYPA were used as positive controls. Gdf11 and Acvr2b mRNA was expressed at low levels in splenic Ter119+ cells isolated from PBS- and RAP-536–treated Hbbth3/+ mice. (T) Erythroid cells, isolated from the spleens of PBS-treated Hbbth3/+ mice and RAP-536–treated mice, were analyzed for Cd71 and Ter119 marker expression before mRNA extraction. (U) mRNA from Ter119+ cells isolated from the spleens of Hbbth3/+ PBS- and RAP-536–treated mice was analyzed for Gdf11 and Acvr2b by qRT-PCR; results show low expression relative to Hprt compared with Tfrc and Gypa in either group. Rho was used as a negative control (data not shown). Data are mean ± standard deviation. *P ≤ .05, **P ≤ .01, ***P ≤ .001, ****P ≤ .0001, Student t test.