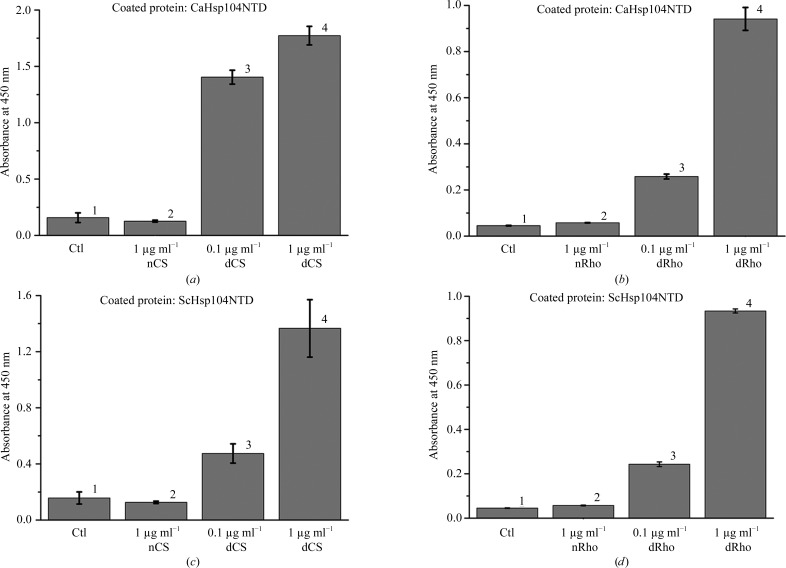
Figure 1.
The yeast Hsp104 N-terminal domain can selectively interact with denatured model proteins but not with native proteins, as shown by ELISA assays. (a) CaHsp104NTD can interact with denatured citrate synthase (CS). CaHsp104NTD was coated onto the ELISA plates and 1 µg ml−1 native CS (nCS) and 0.1 µg ml−1 and 1 µg ml−1 denatured CS (dCS) were added after blocking with 1% BSA. Wells coated with BSA only were utilized as controls (Ctl). After extensive washing, the bound CS can be detected by HRP-conjugated polyclonal CS antibody and the OD450 readings are shown as gray bars. The standard derivations of three independent experiments are shown in the figure. (b) CaHsp104NTD preferably binds denatured rhodanese (dRho) over native rhodanese (nRho). The bound rhodanese can be detected with an HRP-conjugated polyclonal antibody against rhodanese. (c) ScHsp104NTD can interact with denatured citrate synthase but not with native citrate synthase. (d) ScHsp104NTD selectively interacts with denatured rhodanese (dRho) over native rhodanese.