Table 1.
Reaction condition optimization for hydrofluorination of alkyne.a
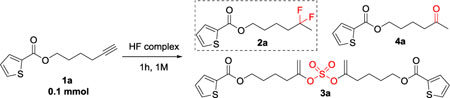 | ||||
|---|---|---|---|---|
| entry | HF complex (A-xHF) |
T (°C) |
solvent | yield (1a /2a /3a /4a)b |
| 1 | 1 equiv KHSO4-13HF | rt | DCE | 75% / 9% / 11% /5% |
| 2 | 1 equiv K2SO4-14HF | rt | DCE | 100% / 0 / 0 / 0 |
| 3 | 1 equiv Py-9HF | rt | DCE | 100% / 0 / 0 / 0 |
| 4 | 1 equiv DMPU-12HF | rt | DCE | 100% / 0 / 0 / 0 |
| 5 | 1 equiv KHSO4-13HF | rt | Toluene | 79% / 8% / 8% / 5% |
| 6 | 1 equiv KHSO4-13HF | rt | HFIP | 29% /23% /20% /28% |
| 7c | 1 equiv K2SO4-14HF | rt | DCE | 100% / 0 / 0 / 0 |
| 8d | 1 equiv K2SO4-14HF | rt | DCE | 100% / 0 / 0 / 0 |
| 9 | 1 equiv KHSO4-13HF | 50 | DCE | 62% /13%/ 16% /9% |
| 10 | 1 equiv DMPU-12HF + 1 equiv KHSO4-13HF | 50 | DCE | 48% /27% /14% /11% |
| 11e | 2 equiv DMPU-12HF | 50 | DCE | 100% / 0 / 0 / 0 |
| 12 | 2 equiv DMPU-12HF + 1 equiv KHSO4-13HF | 50 | Neat | 6% / 65% /12% /16% |
| 13 | 1 equiv DMPU-12HF + 0.5 equiv KHSO4-13HF | 50 | Neat | 46% / 39% / 6% /9% |
| 14 | 2 equiv DMPU-12HF + 0.5 equiv KHSO4-13HF | 50 | Neat | 38 / 46% /6% /10% |
| 15f | 2 equiv DMPU-12HF + 1 equiv KHSO4-13HF | 50 | Neat | 0 / 72% /12% /16% |
Reaction conditions: 1 (0.1 mmol), HF complex (1 equiv based on the complex A-xHF, equivalents of HF are x), solvent (0.1 mL), 1 hour.
NMR yields with benzotrifluoride as internal standard;
1 equiv TFA was added;
1 equiv TfOH was added;
1 equiv KHSO4 was added;
3 h reaction time;
