Abstract
Background
Post-operative recurrence rates are high for hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC). This study aimed to explore the factors associated with post-operative 1-year recurrence rate in patients with HBV-related HCC who had a single small primary tumor (≤3 cm in diameter).
Methods
This was a retrospective study of 203 (training cohort) and 64 (validation cohort) patients newly diagnosed with HBV-related HCC who had a single small primary tumor. The first year of post-operative follow-up was examined. Factors potentially associated with HCC recurrence were identified using Cox regression analyses. A model was constructed based on the factors identified and the prognostic value of the model was evaluated using receiver operating characteristic (ROC) curve analysis and calculation of the area under the ROC curve (AUC).
Results
A history of alcoholism and serum levels of α-fetoprotein, total protein and γ-glutamyl transpeptidase (GGT) were independently associated with 1-year recurrence rate after surgery. A predictive model based on these four factors had an AUC of 0.711 (95% confidence interval, 0.643–0.772) in the training cohort and 0.727 (95% confidence interval, 0.601–0.831) in the validation cohort. The 1-year recurrence rate was significantly lower in the low-risk group than in the high-risk group in both the training cohort (17.0% vs. 49.5%, P < 0.001) and the validation cohort (43.2% vs. 74.1%, P = 0.031).
Conclusion
A history of alcoholism and serum levels of α-fetoprotein, total protein and γ-glutamyl transpeptidase were independently associated with post-operative 1-year recurrence rate in patients with HBV-related HCC who had a single small primary tumor (≤3 cm in diameter).
Keywords: Hepatitis B virus, hepatocellular carcinoma, prognostic factors, recurrence
Introduction
In 2012, approximately 78.25 million people worldwide were diagnosed with hepatocellular carcinoma (HCC) and 74.55 million people died from this cancer [1]. Chinese HCC patients account for more than 50% of all newly diagnosed cases of HCC and all deaths due to HCC [1]. Chronic hepatitis B virus (HBV) infection is a major risk factor for HCC [2]. About 350 million people worldwide suffer from chronic HBV infection and HBV infection is the main cause of HCC in China [3, 4].
The detection rate of small primary HCC has increased in recent years due to improvements in imaging techniques [5–7]. However, disease recurrence commonly occurs after the treatment of small HCCs, with 1-year recurrence rates reported to be 30%–40% [8–10]. Many factors affect the risk of post-operative HCC recurrence, including tumor size, tumor encapsulation, microvascular invasion, liver cirrhosis, serum α-fetoprotein (AFP) level >400 μg/L and use of antiviral drugs [11–14].
Various scoring systems are available for predicting the risk of primary HCC occurrence in Chinese and other populations [15–19]. Nevertheless, HBV-related HCC has a natural history that differs from that of non-HBV-related HCC [3, 4]. Furthermore, prognosis is influenced by whether there is early or late recurrence of HCC after surgical resection of the primary tumor [20, 21]. Currently, there is no scoring system to predict patient survival or the recurrence of HBV-related HCC after treatment in patients with a small primary tumor. Such a scoring system could facilitate close surveillance of patients at high risk of recurrence.
There is controversy regarding the definition of early versus late recurrence of HCC. Park et al. [22] observed that the survival rate of patients who had HCC recurrence within 6 months of liver resection was significantly lower than that of patients who experienced HCC recurrence after 6 months. Therefore, they suggested a time point of 6 months after treatment as a threshold for early/late recurrence. On the other hand, Cheng et al. [23] suggested that recurrence within 2 years after surgery should be considered early recurrence. Guidelines generally recognize the 2-year threshold for early/late recurrence, based on the concept that early recurrence (<2 years) is caused by intrahepatic metastases, whereas late recurrence (>2 years) is caused by multicentric metastases [24]. Nevertheless, Imamura et al. [25] suggested that the first post-operative year was the period in which the risk of HCC recurrence was the highest. Based on the characteristics of the patients (geographical location [Asia] and tumor size), the 1-year threshold was selected for use in the present study.
This study aimed to explore the factors associated with post-operative 1-year recurrence rate in patients with HBV-associated HCC who had a single small (≤3 cm in diameter) primary tumor and to develop a scoring system that could predict the risk of recurrence. It was anticipated that the results would provide a basis for clinicians to determine the post-operative recurrence risk of HCC.
Methods
Study design
This was a retrospective study of patients with HBV-related HCC who had a single small (≤3 cm) primary tumor. All patients were grouped into two cohorts. The training and validation cohorts included patients newly diagnosed with HBV-related HCC and a single small primary tumor at Beijing Ditan Hospital, Capital Medical University (Beijing, China) between January 2012 and December 2014 and between January 2015 and June 2015, respectively.
The study was approved by the ethics committee of Beijing Ditan Hospital, Capital Medical University. The need for individual consent was waived by the committee because of the retrospective nature of the study.
Patient inclusion
The inclusion criteria were as follows: (i) HBV-related HCC; (ii) a single isolated small (≤3 cm in diameter) primary tumor; (iii) 18–75 years of age; and (iv) data were available for at least 1 year of follow-up after surgery. The exclusion criteria included the following items: (i) evidence of hepatitis C virus (HCV) or human immunodeficiency virus (HIV) infection; (ii) severe disease or dysfunction of the heart, lungs, brain, kidneys or other vital organs; (iii) severe mental illness; (iv) pregnancy/lactation; or (v) incomplete clinical data.
For patients who underwent surgery or biopsy, the diagnosis of HCC was based on histopathological examination. For patients who did not undergo biopsy or surgery, the diagnosis of HCC was made clinically according to the clinical symptoms and either at least two imaging modalities indicating HCC (hepatic arteriography, magnetic resonance imaging [MRI], computed tomography [CT] and liver ultrasound) or one imaging modality indicating HCC combined with a serum AFP level ≥400 ng/mL [2].
The patients were treated according to international guidelines [24]. Based on the Chinese guidelines, transarterial chemoembolization (TACE) was used as a locoregional treatment because of the minimal trauma associated with this technique [26]. Blood tests, measurements of HBV-DNA and serum AFP, and liver ultrasonography were performed every 3–6 months after surgery [24, 26].
Outcomes
The analysis was limited to the first year of follow-up after surgery. The outcome was recurrence of HCC during the first year after surgery. Measurements of serum AFP level and liver ultrasonography were carried out every 3–6 months. CT or MRI was performed if necessary. The criteria used to diagnose HCC recurrence were: (i) new lesions observed in the original tumor bed and its surroundings or other parts of the liver; and (ii) the new lesions met the imaging criteria for primary HCC [2].
Collection of clinical data
The following baseline parameters and outcome factors were extracted from the medical records: sex, age, surgical approach, smoking history, history of alcoholism, family history, tumor size, cirrhosis, white blood cell count (WBC), CD8+ lymphocytes, hemoglobin (HGB) level, neutrophil–lymphocyte ratio (NLR), serum levels of alanine transaminase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (GGT), total bilirubin (TBIL), total protein (TP), albumin (ALB), creatinine (Cr) and AFP, prothrombin time (PT), HBV-DNA, Child-Turcotte-Pugh (CTP) score and Model for End-Stage Liver Disease (MELD) score [27]. The reference range for AFP (ARCHITECT AFP Assay, Abbott Laboratories, IL, USA) was 0.89–8.78 ng/mL. Therefore, a serum AFP level >8.78 ng/mL was considered a positive test result.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). Data were tested for normality by the Kolmogorov-Smirnov method. Normally distributed continuous data are expressed as mean ± standard deviation and were analyzed using Student’s t-test. Non-normally distributed continuous data are presented as median (range) and were analyzed using the Mann–Whitney U test. Categorical data are expressed as frequency and were analyzed using the chi-squared test.
First, factors associated with the 1-year recurrence rate were analyzed by univariable Cox regression analyses. Then, factors with P-values <0.05 were included in a multivariable Cox regression analysis (using the forward and maximal-likelihood ratio methods) to establish a Cox proportional hazards regression model. Recurrence rate was analyzed by the Kaplan–Meier method and the log-rank test. The cut-off values for each factor and for the whole model were determined by receiver operating characteristic (ROC) curve analysis with calculation of the Youden index and the diagnostic value of the established model was evaluated using the area under the ROC curve (AUC). Cut-offs for continuous variables were based on the Youden index. A two-sided P-value <0.05 was considered statistically significant.
Results
Baseline characteristics
Patients with evidence of HCV or HIV infection (n = 42), with severe disease or dysfunction of the heart, lungs, brain, kidneys or other vital organs (n = 4), with severe mental illness (n = 2), who were pregnant or breastfeeding (n = 1) or with incomplete clinical data (n = 35) were excluded from the study.
The training cohort included 203 patients, 149 males (73.4%) and 54 females (26.6%), with a mean age of 54.7 ± 9.0 years. The validation cohort included 64 patients, 47 males (73.4%) and 17 females (26.6%), with a mean age of 57.1 ± 9.2 years.
The number of patients with HCC recurrence during the first year after treatment was 66 patients (32.5%) in the training cohort and 36 patients (56.3%) in the validation cohort (Table 1).
Table 1.
Baseline characteristics of patients with a primary single small HBV-associated HCC according to the recurrence status at 1 year
| Training cohort |
Validation cohort |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Recurrence (n = 66) | No recurrence (n = 137) | P | Recurrence (n = 36) | No recurrence (n = 28) | P |
| Agea (years) | 54.9 ± 9.1 | 54.4 ± 9.0 | 0.723 | 58.2 ± 9.1 | 55.6 ± 9.3 | 0.262 |
| Sex (male) | 54 (81.8%) | 95 (69.3%) | 0.060 | 30 (83.3%) | 17 (60.7%) | 0.042 |
| Drinking history (>20 g/day) | 24 (36.4%) | 30 (21.9%) | 0.029 | 18 (50.0%) | 6 (21.4%) | 0.019 |
| Smoking history | 23 (34.8%) | 38 (28.1%) | 0.332 | 13 (36.1%) | 7 (25.0%) | 0.341 |
| Family history | 24 (36.4%) | 37 (27.0%) | 0.173 | 14 (38.9%) | 10 (35.7%) | 0.795 |
| CTP grade | 0.025 | 0.182 | ||||
| A | 37 (56.1%) | 86 (62.8%) | 18 (50.0%) | 20 (71.4%) | ||
| B | 18 (27.3%) | 44 (32.1%) | 11 (20.6%) | 6 (21.4%) | ||
| C | 11 (16.7%) | 7 (5.1%) | 7 (19.4%) | 2 (7.1%) | ||
| Liver cirrhosis | 62 (93.9%) | 126 (91.9%) | 0.300 | 32 (88.9%) | 25 (89.3%) | 0.960 |
| Portal-vein thrombosis and/or vascular invasion | 2 (3.0%) | 3 (2.2%) | 0.717 | 1 (2.8%) | 1 (3.6%) | 0.856 |
| Portal hypertension | 15 (22.7%) | 39 (28.5%) | 0.386 | 12 (33.3%) | 5 (17.9%) | 0.164 |
| Tumor size | 0.577 | 0.355 | ||||
| <2 cm | 36 (54.5%) | 69 (50.4%) | 19 (52.8%) | 18 (64.3%) | ||
| 2–3 cm | 30 (45.5%) | 68 (49.6%) | 17 (47.2%) | 10 (35.7%) | ||
| Treatment | 0.037 | 0.152 | ||||
| RFA | 5 (7.6%) | 21 (15.3%) | 9 (25.0%) | 11 (39.3%) | ||
| TACE | 26 (39.4%) | 31 (22.6%) | 17 (47.2%) | 6 (21.4%) | ||
| RFA+TACE | 28 (42.4%) | 62 (45.3%) | 6 (16.7%) | 6 (21.4%) | ||
| Resection | 7 (10.6%) | 23 (16.8%) | 4 (11.1%) | 3 (10.7%) | ||
| HBV-DNA (positive) | 23 (42.6%) | 36 (33.0%) | 0.232 | 14 (38.9%) | 10 (35.7%) | 0.067 |
| Antiviral drugs | 28 (49.1%) | 63 (52.9%) | 0.635 | 18 (50%) | 16 (57.1%) | 0.570 |
| AFP (positive) | 40 (60.6%) | 53 (38.7%) | 0.003 | 21 (58.3%) | 9 (32.1%) | 0.037 |
| MELD scorea | 5.9 ± 4.5 | 5.1 ± 4.2 | 0.197 | 5.5 ± 2.4 | 4.8 ± 2.3 | 0.276 |
| WBCa (×109/L) | 4.3 ± 2.0 | 4.1 ± 2.0 | 0.409 | 4.1 ± 1.9 | 4.5 ± 1.8 | 0.414 |
| HGBa (g/L) | 130.0 ± 21.5 | 126.3 ± 25.7 | 0.197 | 124.9 ± 22.1 | 127.0 ± 17.0 | 0.681 |
| ALTb (U/L) | 29.7 (21.6, 45.5) | 27.9 (19.0, 41.3) | 0.258 | 28.0 (22.1, 39.2) | 24.3 (19.1, 56.5) | 0.892 |
| TBilb (μmol/L) | 17.1 (12.5, 25.4) | 17.2 (12.5, 25.6) | 0.647 | 17.9 (12.4, 26.5) | 15.5 (11.5, 28.5) | 0.361 |
| GGTb (U/L) | 41.0 (25.5, 79.6) | 34.1 (21.0, 57.4) | 0.023 | 46.1 (26.9, 93.5) | 27.0 (18.0, 40.0) | 0.004 |
| TPb (g/L) | 67.2 (63.7, 69.9) | 69.5 (64.6, 74.7) | 0.032 | 66.5 ± 6.9 | 69.2 ± 8.4 | 0.156 |
| ALBb (g/L) | 36.8 (31.3, 40.9) | 38.20 (32.0, 42.5) | 0.167 | 39.0 (30.5, 41.8) | 40.0 (33.7, 41.8) | 0.477 |
| CRa (μmol/L) | 70.3 ± 16.6 | 69.6 ± 16.0 | 0.762 | 80.2 ± 29.6 | 68.6 ± 16.8 | 0.068 |
| PTa (s) | 13.8 ± 2.8 | 13.5 ± 2.3 | 0.398 | 13.2 ± 2.4 | 12.6 ± 1.5 | 0.234 |
| CD8+ T lymphocytesa | 248.1 ± 135.9 | 321.2 ± 209.8 | 0.040 | 332.2 ± 186.7 | 421.6 ± 349.6 | 0.327 |
These values are presented as mean ± standard deviation.
These values are presented as range followed by 95% confidential interval in parentheses; other values are presented as numbers of patients followed by percentages in parentheses.
CTP, Child-Turcotte-Pugh; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; HBV, hepatitis B virus; AFP, α-fetoprotein; MELD, Model for End-Stage Liver Disease; WBC, white blood cells; NLR, neutrophil–lymphocyte ratio; PLT, platelets; ALT, alanine transaminase; TBIL, total bilirubin; GGT, γ-glutamyl transpeptidase; TP, total proteins; ALB, albumin; CR, creatinine; PT, prothrombin time.
In the training cohort, patients who experienced HCC recurrence within 1 year had a higher rate of heavy drinking (P = 0.029), higher CTP grades (P = 0.025), a higher rate of treatment with TACE (P = 0.037), higher GGT levels (P = 0.023), lower TP levels (P = 0.032) and higher rates of AFP positivity (P = 0.003) than patients who did not have disease recurrence.
In the validation cohort, patients with HCC recurrence within 1 year were more commonly male (P = 0.042) and had a higher rate of heavy drinking (P = 0.019), higher GGT levels (P = 0.004) and higher rates of AFP positivity (P = 0.037) than patients without disease recurrence.
For patients without a history of heavy drinking, HBV-DNA level was not associated with outcome in either the training cohort (P = 0.903, chi-squared test) or the validation cohort (P = 0.744, chi-squared test).
Predictors of post-operative recurrence of HCC
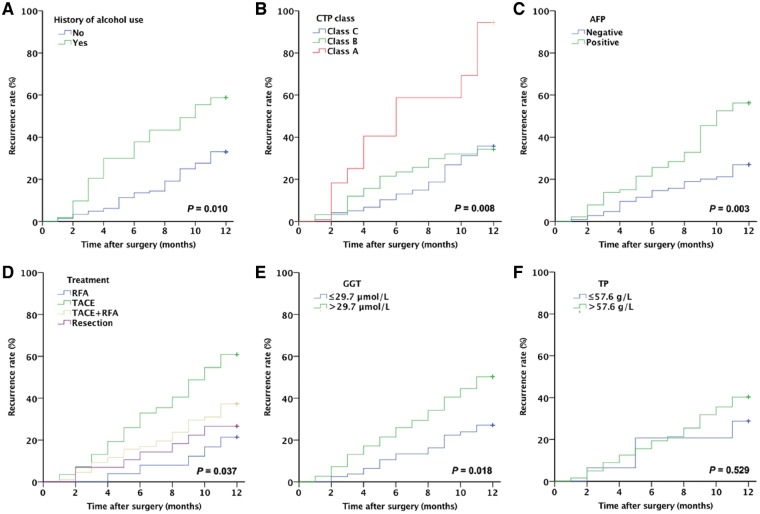
The 1-year HCC recurrence rate was higher in patients with a history of alcoholism than in those without a history of alcoholism (44.4% vs. 28.2%, P = 0.01; Figure 1A). The 1-year HCC recurrence rate was significantly higher in patients with CTP grade C than in those with CTP grade A (61.1% vs. 30.1%, P = 0.008; Figure 1B). AFP-positive patients had a higher 1-year HCC recurrence rate than AFP-negative patients (43.0% vs. 23.6%, P = 0.003; Figure 1C).
Figure 1.
History of alcohol use, CTP class, AFP levels, GGT levels and treatment approach are associated with the 1-year recurrence rate of a single small HBV-related HCC. The curves were constructed using the Kaplan–Meier method and analyzed using the log-rank test for 1-year recurrence rate of single small HBV-related HCC (A) according to the history of heavy alcohol use; (B) according to CTP grade; (C) according to serum AFP positivity; (D) according to serum GGT levels; (E) according to serum TP level; and (F) according to the treatment approach. CTP, Child-Turcotte-Pugh grade; AFP, α-fetoprotein; GGT, γ-glutamyl transpeptidase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; TP, total protein.
The cut-off values for GGT and TP were determined based on the Youden index. The 1-year HCC recurrence rate was significantly higher in patients with GGT >29.7 μmol/L than in those with GGT ≤29.7 μmol/L (P = 0.018; Figure 1D). However, the 1-year HCC recurrence rate did not differ significantly between patients with TP >57.6 g/L and those with TP ≤57.6 g/L (P = 0.529; Figure 1E).
The 1-year recurrence rate showed significant variation depending on the treatment used after the diagnosis of HCC (P = 0.037). The lowest 1-year recurrence rate (19.2%) was observed in patients who underwent radiofrequency ablation (RFA), while the highest 1-year recurrence rate (45.6%) was observed in patients treated with TACE (P = 0.019). The 1-year recurrence rate after liver resection (23.3%) was not significantly difference from that after RFA (P = 0.654; Figure 1F).
These results indicate that certain demographic and clinical characteristics are associated with HCC recurrence within 1 year of surgery.
Establishment of the scoring model
The results of the univariable Cox regression analyses showed that a history of alcoholism, serum levels of GGT, AFP and TP, and blood levels of CD8+ T lymphocytes were associated with 1-year HCC recurrence rate. The multivariable Cox regression analysis indicated that a history of alcoholism (HR, 1.813; 95% confidence interval [CI], 1.081–3.039; P = 0.024), serum GGT level (HR, 1.006; 95% CI, 1.002–1.010; P = 0.003), serum TP level (HR, 0.960; 95% CI, 0.929–0.992; P = 0.014) and serum AFP level (HR, 2.073; 95% CI, 1.232–3.487; P = 0.006) were independently associated with 1-year recurrence rate in patients with HBV-related HCC who had a single small primary tumor (Table 2).
Table 2.
Cox regression analyses of the factors associated with the 1-year recurrence rate in patients with a single small HCC
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | HR | P | 95% CI | HR | P | 95% CI |
| Sex | 0.540 | 0.053 | 0.289–1.009 | |||
| Age | 0.997 | 0.812 | 0.971–1.024 | |||
| Alcoholism history | 1.889 | 0.013 | 1.143–3.120 | 1.813 | 0.024 | 1.081–3.039 |
| WBC | 1.049 | 0.424 | 0.934–1.178 | |||
| NLR | 1.028 | 0.420 | 0.961–1.100 | |||
| PLT | 0.997 | 0.299 | 0.992–1.002 | |||
| ALT | 1.001 | 0.498 | 0.998–1.003 | |||
| GGT | 1.008 | 0.000 | 1.004–1.012 | 1.006 | 0.003 | 1.002–1.010 |
| TBIL | 1.005 | 0.334 | 0.995–1.015 | |||
| TP | 0.970 | 0.050 | 0.941–1.000 | 0.960 | 0.014 | 0.929–0.992 |
| HBV-DNA | 1.439 | 0.186 | 0.839–2.468 | |||
| PT | 1.042 | 0.387 | 0.949–1.143 | |||
| AFP | 1.809 | 0.019 | 1.250–3.359 | 2.073 | 0.006 | 1.232–3.487 |
| CD8+ T lymphocytes | 0.998 | 0.048 | 0.996–1.000 | |||
HR, hazard ratio; 95% CI, 95% confidence interval; WBC, white blood cells; NLR, neutrophil–lymphocyte ratio; PLT, platelets; ALT, alanine transaminase; GGT, γ-glutamyl transpeptidase; TBIL, total bilirubin; TP, total proteins; HBV, hepatitis B virus; PT, prothrombin time; AFP, α-fetoprotein.
The Cox regression equation was: recurrence score (RS) = 0.595 × history of alcoholism (yes, 1; no, 0) + 0.006 × GGT − 0.041 × TP + 0.729 × AFP (positive, 1; negative, 0). The optimal cut-off value (specificity + sensitivity − 1) was calculated as −1.67. Therefore, RS = −1.67, RS < −1.67 and RS > −1.67 was taken to indicate average, low and high risk of HCC recurrence during the first year after surgery, respectively.
Prognostic performance of the RS
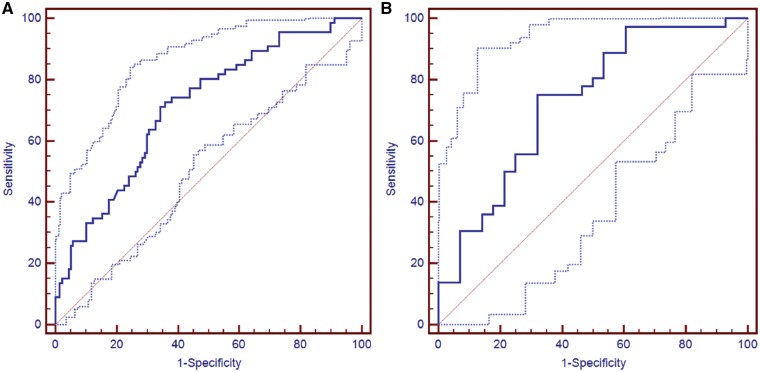
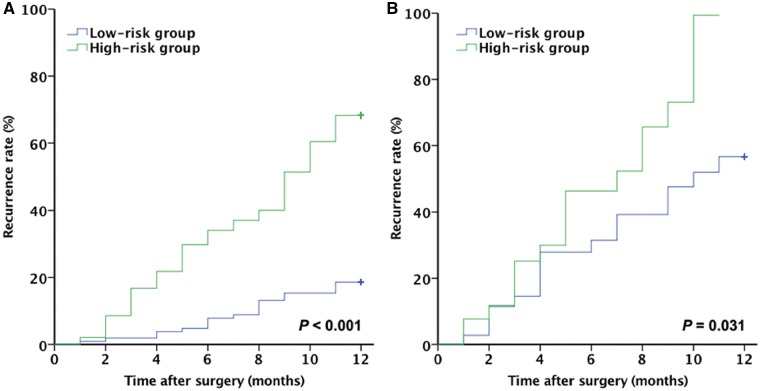
The AUC of the RS was 0.711 (95% CI, 0.643–0.772) in the training cohort and 0.727 (95% CI, 0.601–0.831) in the validation cohort (Figure 2). Patients in the training and validation cohorts were divided into high-risk and low-risk groups according to the optimal cut-off value for RS (−1.67). There were 106 patients with low HCC recurrence risk and 97 patients with high HCC recurrence risk in the training cohort, and the 1-year recurrence rate differed significantly between them (17.0% vs. 49.5%, P < 0.001; Figure 3A). In the validation cohort, the 1-year recurrence rate was 43.2% in the low-risk group and 74.1% in the high-risk group (P = 0.031; Figure 3B). These results indicate that a prognostic model can be built and used clinically to predict the prognosis of patients with HBV-related HCC who receive surgical treatment for a single small primary tumor.
Figure 2.
The recurrence score has a good prognostic performance in predicting the 1-year recurrence rate of a single small HBV-associated HCC. The patients in the training and validation cohorts were divided into high-risk and low-risk groups according to the cut-off value (−1.67) of the recurrence score. The prognostic performance was analyzed using ROC curve analysis; (A) the training cohort; (B) the validation cohort. HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ROC, receiver operating characteristic; CI, confidence interval.
Figure 3.
The survival rate differed between the low-risk and high-risk recurrence groups. The curves were constructed using the Kaplan–Meier method and analyzed using the log-rank test. One-year recurrence rates for the high-risk and low-risk groups are based on the recurrence score in (A) the training cohort and (B) the validation cohort
Discussion
The results showed that a history of alcoholism and serum levels of AFP, TP and GGT were independently associated with the 1-year recurrence rate of HBV-related HCC in patients treated for a single small primary tumor. The RS could provide a basis for clinicians to determine the post-operative HCC recurrence risk in these patients.
The factors influencing the post-operative recurrence of primary HBV-related HCC include tumor factors, peripheral vascular factors and liver function. Cheng et al. [23] reported that the independent risk factors for early recurrence after liver resection were tumor size >5 cm, tumor without a capsule and microvascular invasion, while the independent risk factors for late recurrence were cirrhosis and AFP >400 μg/L. Truant et al. [28] suggested that portal-vein invasion and tumor size >8 cm were independent risk factors for mortality in patients with HCC. Hirokawa et al. [29] concluded that HCC recurrence within 6 months after liver resection was associated with a lower survival rate. Moreover, in their study, vascular invasion and indocyanine green retention rate at 15 min (ICGR15) ≥16% were considered to be independent risk factors for early post-operative recurrence. Patients with HBV-related HCC who had a single small primary tumor were included in the present study; the results showed that AFP, GGT and a history of alcoholism were independent risk factors for HCC recurrence within 1 year after surgery, while TP was a protective factor. These results are consistent with those reported in previous studies [23, 28, 29]. These factors were used to build a scoring system that could allow closer surveillance of patients at higher risk of recurrence and earlier intervention to improve their prognosis.
Serum AFP level is an important parameter used in the detection of HCC and many studies have found that AFP is also a prognostic indicator in patients with HCC. Marubashi et al. [30] showed that the presence of AFP mRNA-expressing cells was an independent risk factor for post-operative recurrence in patients who received a liver transplant. Lu et al. [31] reported that AFP played an important role in promoting HCC metastasis. Kanda et al. [32] suggested that patients with HCC and an AFP level >100 μg/L were more prone to having post-operative recurrence and metastasis of HCC. Lee et al. [33] indicated that AFP and DCP levels predicted the survival of patients after TACE treatment. The present study confirmed that AFP levels were significantly increased in patients with HBV-associated HCC and a single small primary tumor who experienced recurrence during the first year after treatment than in those without recurrence. Furthermore, AFP was an independent risk factor for 1-year HCC recurrence in these patients.
Long-term heavy alcohol consumption can directly or indirectly damage the liver, causing hepatic fibrosis and liver cancer. Chavez et al. [34] confirmed that long-term chronic overdose of ethanol in mouse models could reduce the level of insulin-like growth factor-1 (IGF-1), affecting the proliferation of normal hepatocytes and promoting tumorigenesis. Purohit et al. [35] indicated that smoking and alcoholism promoted the occurrence of cancer. Takeshita et al. [36] showed that high doses of alcohol were associated with HCC. In the present study, a history of alcoholism was associated with post-operative recurrence in patients with HBV-associated HCC who had a single small primary tumor.
In normal individuals, GGT is mainly distributed in the cytoplasm of hepatocytes and intrahepatic bile duct epithelial cells. Thus, serum GGT is often increased in patients with non-alcoholic fatty liver disease and might be a surrogate marker of the association between metabolic factors and liver damage [37, 38]. A recent study performed in patients with HBV from Taiwan, China, showed that metabolic risk factors increased the risk of HCC development in the setting of chronic HBV hepatitis [39]. Investigations have shown that GGT levels are associated with the occurrence and prognosis of HCC [40–43]. The present study found that GGT levels were positively associated with the 1-year recurrence rate of single small HCCs, which is consistent with the results of the above studies.
TP mainly includes globulin and albumin. Deng et al. [44] showed that the albumin/globulin ratio was associated with the prognosis of HCC. Albumin and globulin are both secreted by the liver and the levels of these proteins in the blood represent the liver status and are associated with HCC prognosis [45, 46]. In the present study, TP level was an indicator of HCC recurrence in patients with small HCC, implying that TP is associated with the prognosis of HCC regardless of globulin or albumin reductions.
Many scoring systems are available for predicting the risk of HCC occurrence [15–19]. Nevertheless, HBV-related HCC has a specific natural history that differs from that of non-HBV-related HCC [3, 4]. The prognosis of HCC is also affected by whether early or late recurrence occurs after resection [20, 21]. The present study proposes an innovative scoring system that is specific to patients with HBV-related HCC who have a single small tumor. Additional studies are necessary to validate and improve this scoring system, for example by the inclusion of additional factors. Previous studies have reported that hyaluronic acid levels, the viral load of HBV and the liver inflammatory state are associated with HCC recurrence [47, 48], but these factors were not assessed in the present study.
This study also has some limitations. First, the study did not consider the tumor location, distance between the tumor and the portal vein, and the pathological features of the tumor. However, our research focused on serum markers that are easy to obtain in the clinical setting and the final model still had a high diagnostic value after validation. Second, the sample size of this study was small and the 95% CI of the AUC was wide. Although a large number of patients with HCC are seen at our institution, the present study aimed to establish a model specifically for patients with small HCCs, which limited the sample size. The sample size should be expanded in a future prospective cohort study. Third, the follow-up was only 1 year and the recurrence rates at 2 and 3 years were not available. Nevertheless, the recurrence rate was already high at 1 year (>30%), highlighting that early prevention and treatment are important for the survival of these patients. Fourth, the follow-up was carried out every 3–6 months or more frequently in cases of elevated AFP level or abnormal liver ultrasound, but the follow-up interval was not controlled due to the retrospective nature of the study; this may have introduced a follow-up bias. The recurrence rates for the two cohorts were significantly different. The hospital moved to a new site in 2008 and there were not as many patients in the first few years as in recent years. In addition, the new hospital has received more non-resident patients and patients with a more severe disease status. Finally, the model was based on patients from Beijing and its surroundings, so studies of other geographical areas are needed. In addition, no assessment of model calibration was performed because of the small sample size.
Radical treatments of HCC include resection and RFA. TACE is considered a palliative treatment and cannot achieve a curative outcome. Nevertheless, some patients in our study received TACE as the primary treatment for a variety of reasons, usually poor liver function and contraindications to surgery. This therapeutic approach is supported by the Chinese guidelines for the treatment of HCC [26]. The purpose of the present study was to explore the factors affecting post-operative 1-year HCC recurrence in patients with HBV-associated HCC who had a single small (≤3 cm) primary tumor and to suggest a scoring system for the prediction of recurrence. In the present study, the criteria for recurrence included the detection of new lesions suggestive of liver cancer in and around the original lesion or in other parts of the liver during follow-up. Based on this definition, the original lesion did not have to be removed.
Conclusions
A history of alcoholism and serum levels of AFP, TP and GGT were independently associated with the 1-year recurrence rate of HBV-related HCC in patients with a single small (≤3 cm) primary tumor. Despite its limitations, the RS could provide an estimation of the post-operative 1-year HCC recurrence risk in these patients, but the score has to be validated in larger cohorts.
Authors’ contributions
Z.Y.Y., X.B.W., and Y.Y.J. conceived of and designed the project. L.L.H., X.L.L., S.Z., and M.G.L collected the data. L.L.H. and X.L.L. analyzed the data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Beijing Municipal Science and Technology Commission [No. Z171100001017082], the Special Fund of Capital Health Research and Development [No. 2016–2-2171], the Fund for Beijing Science & Technology Development of TCM [No. JJ2016-14] and the Science and Technology Project of Beijing Municipal Education Commission [No. SQKM201610025026].
Acknowledgements
Thanks to the staff of the Medical Records Room of Beijing Ditan Hospital.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1. Torre LA, Bray F, Siegel RL. et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanaka M, Katayama F, Kato H. et al. Hepatitis B and C virus infection and hepatocellular carcinoma in China: a review of epidemiology and control measures. J Epidemiol 2011;21:401–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu RX, Seto WK, Lai CL. et al. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver 2016;10:332–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu MH, Kim JH, Yoon JH. et al. Small (</=1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 2014;271:748–60. [DOI] [PubMed] [Google Scholar]
- 6. Di Martino M, Di Miscio R, De Filippis G. et al. Detection of small (</=2 cm) HCC in cirrhotic patients: added value of diffusion MR-imaging. Abdom Imaging 2013;38:1254–62. [DOI] [PubMed] [Google Scholar]
- 7. Dong Y, Wang WP, Mao F. et al. Application of imaging fusion combining contrast-enhanced ultrasound and magnetic resonance imaging in detection of hepatic cellular carcinomas undetectable by conventional ultrasound. J Gastroenterol Hepatol 2016;31:822–8. [DOI] [PubMed] [Google Scholar]
- 8. Tung-Ping Poon R, Fan ST, Wong J.. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reig M, Marino Z, Perello C. et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719–26. [DOI] [PubMed] [Google Scholar]
- 10. Portolani N, Coniglio A, Ghidoni S. et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg 2006;243:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ou DP, Yang LY, Huang GW. et al. Clinical analysis of the risk factors for recurrence of HCC and its relationship with HBV. World J Gastroenterol 2005;11:2061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Y, Shen Z, Zhu Z. et al. Clinical values of AFP, GPC3 mRNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon 2011;11:195–9. [PMC free article] [PubMed] [Google Scholar]
- 13. Wu CY, Chen YJ, Ho HJ. et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012;308:1906–14. [DOI] [PubMed] [Google Scholar]
- 14. Camma C, Cabibbo G, Craxi A.. Direct antiviral agents and risk for HCC early recurrence: much ado about nothing. J Hepatol 2016;65:861–2. [DOI] [PubMed] [Google Scholar]
- 15. Wong VW, Chan SL, Mo F. et al. Clinical scoring system to predict hepatocellular carcinoma in chronic hepatitis B carriers. J Clinc Oncol 2010;28:1660–5. [DOI] [PubMed] [Google Scholar]
- 16. Yang HI, Yuen MF, Chan HL. et al. Risk estimation for hepatocellular carcinoma in chronic hepatitis B (REACH-B): development and validation of a predictive score. Lancet Oncol 2011;12:568–74. [DOI] [PubMed] [Google Scholar]
- 17. Wong GL, Chan HL, Wong CK. et al. Liver stiffness-based optimization of hepatocellular carcinoma risk score in patients with chronic hepatitis B. J Hepatol 2014;60:339–45. [DOI] [PubMed] [Google Scholar]
- 18. Michikawa T, Inoue M, Sawada N. et al. Development of a prediction model for 10-year risk of hepatocellular carcinoma in middle-aged Japanese: the Japan Public Health Center-Based Prospective Study Cohort II. Prev Med 2012;55:137–43. [DOI] [PubMed] [Google Scholar]
- 19. Flemming JA, Yang JD, Vittinghoff E. et al. Risk prediction of hepatocellular carcinoma in patients with cirrhosis: the ADRESS-HCC risk model. Cancer 2014;120:3485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kudo M. Early hepatocellular carcinoma: definition and diagnosis. Liver Cancer 2013;2:69–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kao WY, Chao Y, Chang CC. et al. Prognosis of early-stage hepatocellular carcinoma: the clinical implications of substages of Barcelona clinic liver cancer system based on a cohort of 1265 patients. Medicine (Baltimore) 2015;94:e1929.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park JH, Koh KC, Choi MS. et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg 2006;192:29–33. [DOI] [PubMed] [Google Scholar]
- 23. Cheng Z, Yang P, Qu S. et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines)—Hepatobiliary Cancers Version 4.2017. Fort Washington: National Comprehensive Cancer Network, 2017.
- 25. Imamura H, Matsuyama Y, Tanaka E. et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200–7. [DOI] [PubMed] [Google Scholar]
- 26. Kudo M. Clinical practice guidelines for hepatocellular carcinoma differ between Japan, United States, and Europe. Liver Cancer 2015;4:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huo TI, Lin HC, Wu JC. et al. Proposal of a modified Child-Turcotte-Pugh scoring system and comparison with the model for end-stage liver disease for outcome prediction in patients with cirrhosis. Liver Transpl 2006;12:65–71. [DOI] [PubMed] [Google Scholar]
- 28. Truant S, Boleslawski E, Duhamel A. et al. Tumor size of hepatocellular carcinoma in noncirrhotic liver: a controversial predictive factor for outcome after resection. Eur J Surg Oncol 2012;38:1189–96. [DOI] [PubMed] [Google Scholar]
- 29. Hirokawa F, Hayashi M, Asakuma M. et al. Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016;25:24–9. [DOI] [PubMed] [Google Scholar]
- 30. Marubashi S, Dono K, Nagano H. et al. Detection of AFP mRNA-expressing cells in the peripheral blood for prediction of HCC recurrence after living donor liver transplantation. Transplant Int 2007;20:576–82. [DOI] [PubMed] [Google Scholar]
- 31. Lu Y, Zhu M, Li W. et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med 2016;20:549–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kanda M, Tateishi R, Yoshida H. et al. Extrahepatic metastasis of hepatocellular carcinoma: incidence and risk factors. Liver Int 2008;28:1256–63. [DOI] [PubMed] [Google Scholar]
- 33. Lee YK, Kim SU, Kim DY. et al. Prognostic value of alpha-fetoprotein and des-gamma-carboxy prothrombin responses in patients with hepatocellular carcinoma treated with transarterial chemoembolization. BMC Cancer 2013;13:5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chavez PR, Lian F, Chung J. et al. Long-term ethanol consumption promotes hepatic tumorigenesis but impairs normal hepatocyte proliferation in rats. J Nutr 2011;141:1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Purohit V, Rapaka R, Kwon OS. et al. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci 2013;92:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Takeshita T, Yang X, Inoue Y. et al. Relationship between alcohol drinking, ADH2 and ALDH2 genotypes, and risk for hepatocellular carcinoma in Japanese. Cancer Lett 2000;149:69–76. [DOI] [PubMed] [Google Scholar]
- 37. Pang Q, Bi JB, Wang ZX. et al. Simple models based on gamma-glutamyl transpeptidase and platelets for predicting survival in hepatitis B-associated hepatocellular carcinoma. Onco Targets Ther 2016;9:2099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dong ZR, Zou J, Sun D. et al. Preoperative albumin-bilirubin score for postoperative solitary hepatocellular carcinoma within the Milan criteria and Child-Pugh A Cirrhosis. J Cancer 2017;8:3862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yu MW, Lin CL, Liu CJ. et al. Influence of metabolic risk factors on risk of hepatocellular carcinoma and liver-related death in men with chronic hepatitis B: a large cohort study. Gastroenterology 2017;153:1006–17.e5. [DOI] [PubMed] [Google Scholar]
- 40. Zhang JB, Chen Y, Zhang B. et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol 2011;23:787–93. [DOI] [PubMed] [Google Scholar]
- 41. Song P, Inagaki Y, Wang Z. et al. High levels of gamma-glutamyl transferase and indocyanine green retention rate at 15 min as preoperative predictors of tumor recurrence in patients with hepatocellular carcinoma. Medicine (Baltimore) 2015;94:e810.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang Z, Ye P, Xu Q. et al. Elevation of serum GGT and LDH levels, together with higher BCLC staging are associated with poor overall survival from hepatocellular carcinoma: a retrospective analysis. Discov Med 2015;19:409–18. [PubMed] [Google Scholar]
- 43. Chen D, Wang R, Meng X. et al. Prognostic value of serum gamma-glutamyl transferase in unresectable hepatocellular carcinoma patients treated with transcatheter arterial chemoembolization combined with conformal radiotherapy. Oncol Lett 2014;8:2298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deng Y, Pang Q, Miao RC. et al. Prognostic significance of pretreatment albumin/globulin ratio in patients with hepatocellular carcinoma. Onco Targets Ther 2016;9:5317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hiwatashi K, Ueno S, Sakoda M. et al. Problems of long survival following surgery in patients with NonBNonC-HCC: comparison with HBV and HCV related-HCC. J Cancer 2015;6:438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim JS, Yoon SK, Kim JA. et al. Long-term survival in a patient with ruptured hepatocellular carcinoma. Korean J Intern Med 2009;24:63–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xia F, Lai EC, Lau WY. et al. High serum hyaluronic acid and HBV viral load are main prognostic factors of local recurrence after complete radiofrequency ablation of hepatitis B-related small hepatocellular carcinoma. Ann Surg Oncol 2012;19:1284–91. [DOI] [PubMed] [Google Scholar]
- 48. Wu JC, Huang YH, Chau GY. et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol 2009;51:890–7. [DOI] [PubMed] [Google Scholar]