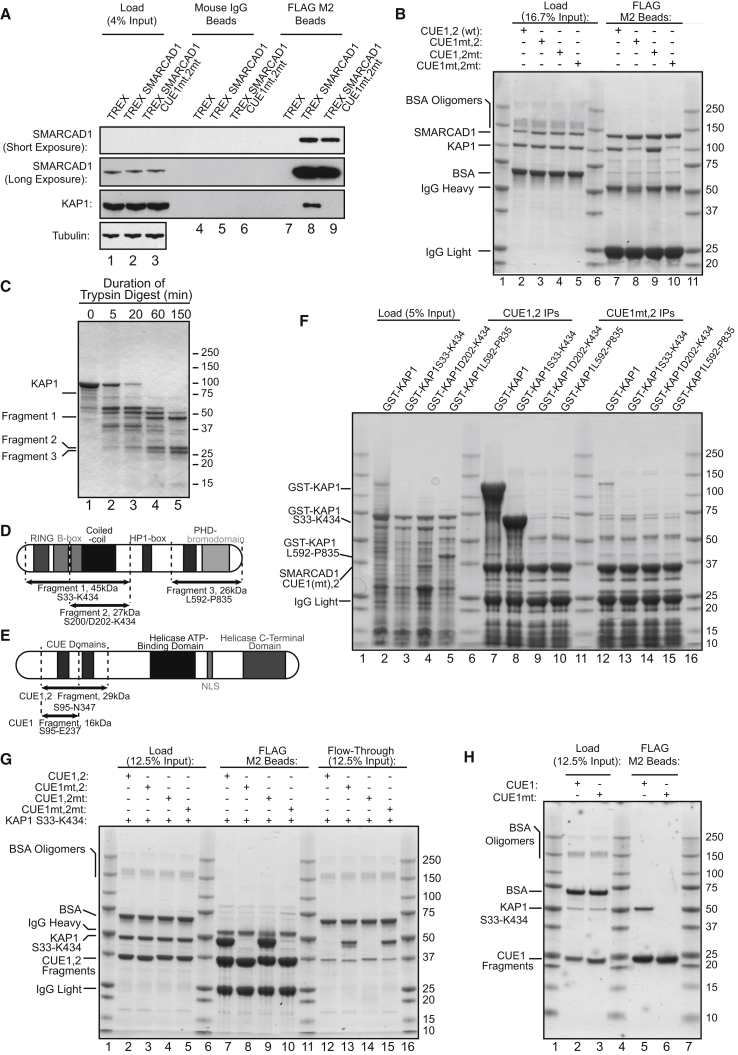
Figure 1.
SMARCAD1 CUE1 and KAP1 RBCC Are Necessary and Sufficient for a Direct Interaction
(A) Wild-type, but not SMARCAD1 CUE1mt,2mt, coimmunoprecipitates KAP1 (compare lanes 8 and 9) in human cells.
(B) The SMARCAD1-KAP1 interaction reconstituted with nonubiquitylated, purified proteins, expressed in E. coli. Mutation of SMARCAD1 CUE1 significantly compromises KAP1 binding (compare lanes 7 and 8).
(C) Limited tryptic proteolysis of purified recombinant KAP1 yields three main fragments relatively resistant to trypsin.
(D) Depiction of the three trypsin-resistant KAP1 fragments, mapped by Edman degradation and intact molecular weight mass spectrometry.
(E) Schematic representation of SMARCAD1 CUE1,2 and CUE1 fragments.
(F) Immobilized SMARCAD1 CUE1,2 enriches for full-length KAP1 (lane 7) and KAP1 S33-K434 (i.e., fragment 1, lane 8), which spans the RBCC domain, from E. coli extract. Binding is specific—mutation of the CUE1 abrogates the interaction (lanes 12 and 13).
(G) The SMARCAD1-KAP1 interaction recapitulated in vitro with SMARCAD1 CUE1,2 and KAP1 S33-K434 depends on functional CUE1 (compare lane 7 with 8 and 10).
(H) SMARCAD1 CUE1 and the KAP1 RBCC are necessary and sufficient (also see Figure S2A) for SMARCAD1-KAP1 interaction.