Figure 2.
A Structural Model of the KAP1 RBCC ΔBBX1-SMARCAD1 CUE1,2 Complex
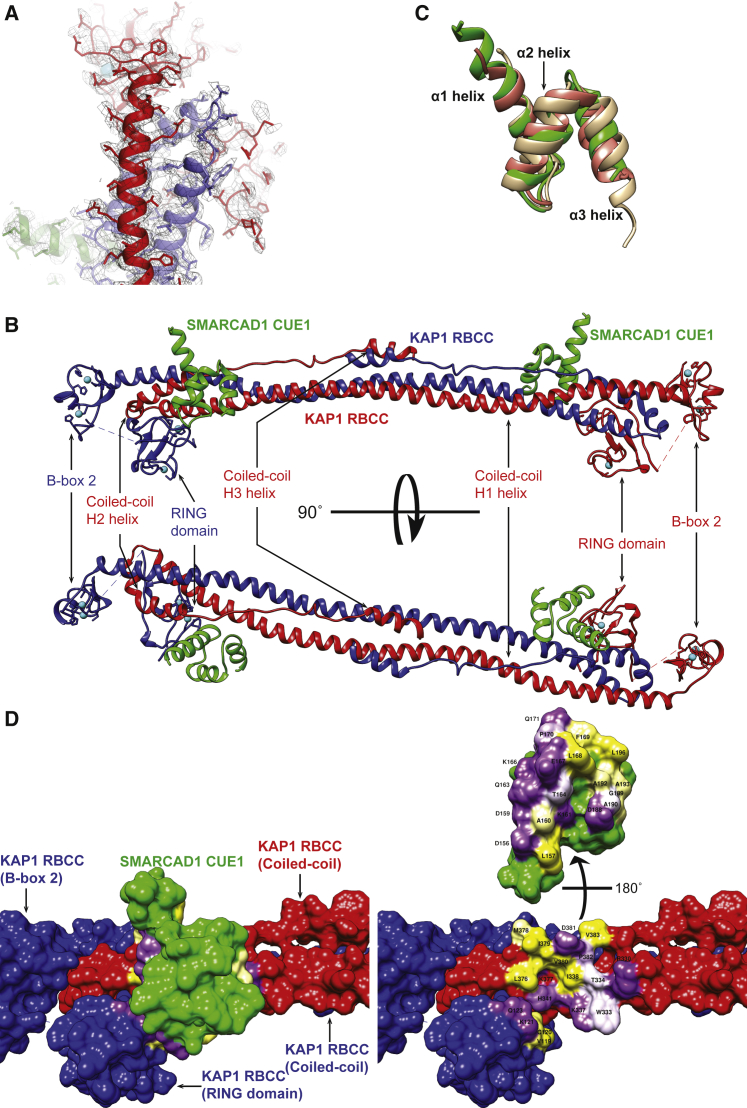
(A) Electron density map of the KAP1 RBCC ΔBBX1-SMARCAD1 CUE1,2 complex (tetragonal form). One KAP1 RBBC chain is colored red, the other blue, and the CUE1 domain green.
(B) KAP1 RBCC homodimerizes in an antiparallel fashion, mediated by the coiled-coil domains. The RING and B-box domains are located at either end of the coiled coil. A SMARCAD1 CUE1 domain binds to each end of the KAP1 RBCC dimer by recognizing an exposed surface of the coiled-coil domain. Domains are colored as in (A).
(C) SMARCAD1 CUE1 (green) resembles canonical CUE domains. The CUE domains of CUE2p (tan) and gp78 (salmon) are superimposed.
(D) The SMARCAD1 CUE1-KAP1 RBCC interaction surface. On the right, CUE1 domain has been rotated 180° off KAP1. Residues involved in the interaction are labeled and colored by hydrophobicity: yellow, hydrophobic; white, hydroneutral; and purple, hydrophilic.