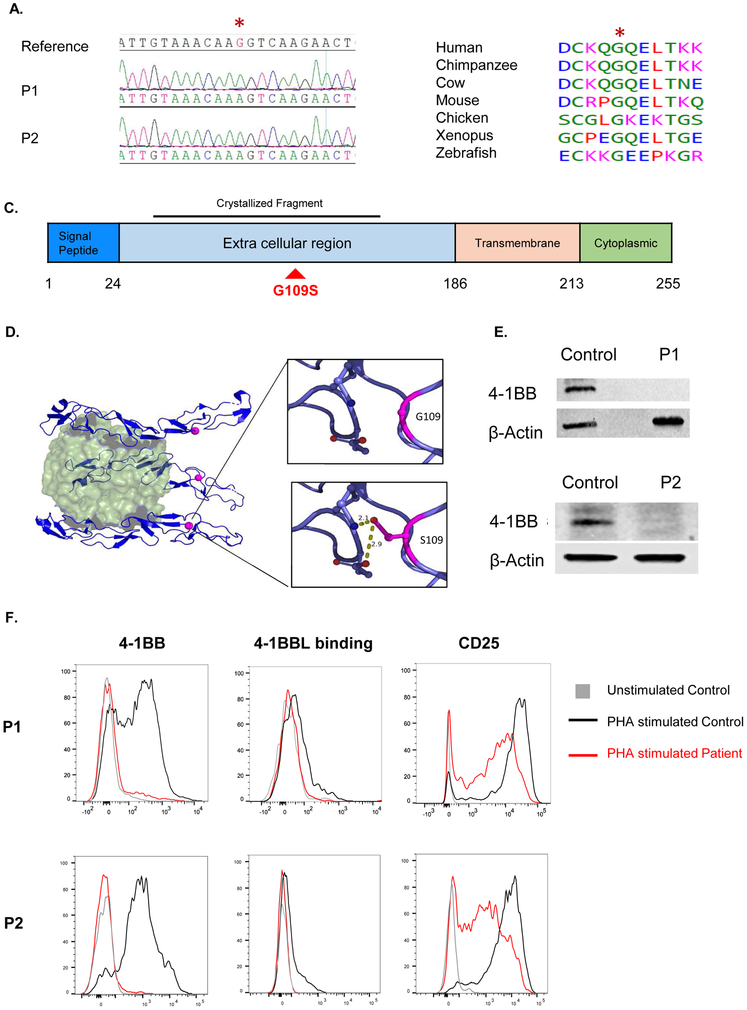
Figure 2. The 4-1BBG109S mutation and its impact on 4-1BB expression.
A. Sanger sequencing around the missense TNFRSF9 mutation (c.325G>A: p.Gly109Ser) in a reference control and patients. The mutated nucleotide is indicated in red and by an asterisk. B. Evolutionary conservation of the S109 a.a. in 4-1BB. Protein sequence orthologous to human 4-1BB was aligned in six non-human vertebrate species, all of which shared the p.S109 amino acid. C. Linear schematic of 4-1BB showing its domains and the location of the G109S mutation (red) in the extracellular domain. The 4-1BB fragment crystallized in complex with 4-1BB (PDB:6PCR) is indicated D. Ribbon diagram of the 4-1BB extracellular in complex 4-1BBL. The 4-1B trimer (blue) is shown bound to its trimeric ligand 4-1BBL (green). The red dot corresponds to the location of the mutated G109 residue. The insets illustrate the potential of the mutated S109 residue to form hydrogen bonds with neighboring polar residues. E. Immunoblot analysis of PHA stimulated PBMCs from both patients and controls using a polyclonal antibody directed against the intracellular domain of 4-1BB. Actin was used as loading control. F. FACS analysis of 4-1BB expression (left), 4-1BBL binding (center) and CD25 expression(right) in PHA stimulated CD8+ T cells from the patients and a control. Similar results were obtained from two independent experiments in E and F.