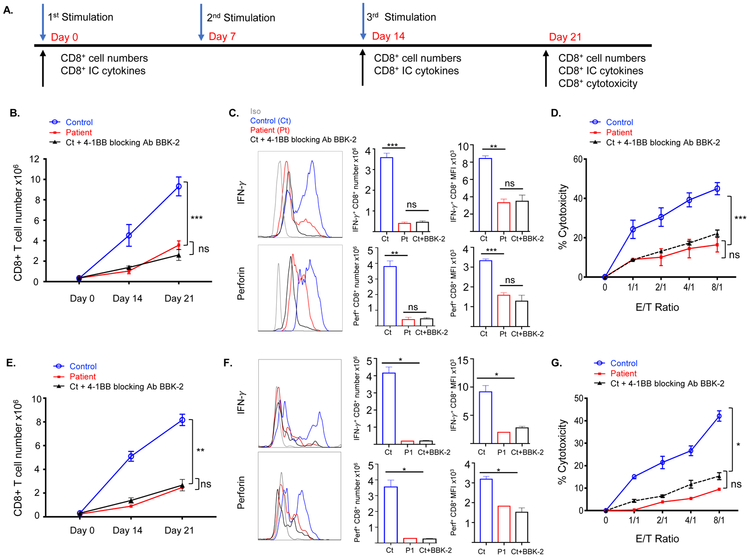
Figure 3. The 4-1BBG109S mutation Impairs the generation of allo- and EBV-specific CD8+ T cells.
A. Experimental protocol for the generation and testing of cytotoxic CD8+ T cells against EBV-B cells. B. CD8+ T cell numbers on days 0, 14 and day 21 post-stimulation with allogeneic EBV-B cells of PBMCs from patients (n=2) controls (n=4) and controls with addition of anti-4-1BB blocking antibody BBK-2 (n=2). C. Representative FACS analysis (left) quantitative analysis (center) and MFI (right) of CD8+IFN-γ+ and CD8+Perforin+ cells on Day 14 post stimulation of PBMCs stimulated with allogeneic EBV-B cells as described in B. D. Cytotoxic activity of CD8+ T cells against the stimulatory allogeneic EBV B cells. CD8+ T cells were purified on Day 21 from cultures of stimulated PBMCs from patients (n=2) controls (n=4). The effect of anti-4-1BB blocking antibody BBK-2 was examined in 2 of the 4 controls (n=2). E-G. CD8+ T cell numbers (F) numbers of CD8+IFN-γ+ and CD8+Perforin+ cells (H) and cytotoxicity of CD8+ T cells (G) on day 21 post-stimulation of PBMCs from P1 with HLA-matched EBV-B cells and of normal PBMCs stimulated with autologous EBV-B cells without or with addition of anti-4-1BB blocking antibody BBK-2 (n=4 and n=2, respectively). We were able to study P1 CD8+ T cell cytotoxicity against HLA-matched EBV-B cell targets once, because the patient underwent HSCT. Symbols and bars in B, D, E and G and columns and bars in C and F represent mean and SEM. *= p<0.05, ** p<0.01, *** p<0.001, ns= not significant.