Figure 2. Grp170 prevents formation of aggregated Akita in vitro.
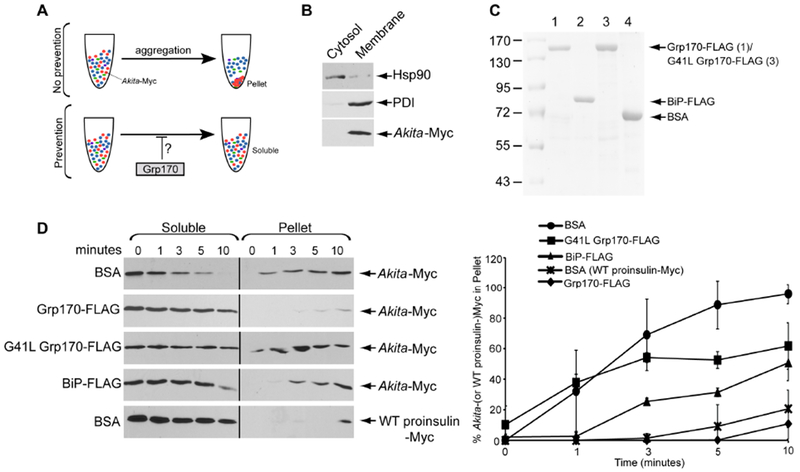
A. Schematic of the in vitro aggregation assay. (top) When Akita-Myc in a membrane extract aggregates, it forms a pellet after centrifugation. (bottom) However, if the extract containing Akita-Myc was incubated with Grp170, aggregation is prevented. Thus Akita-Myc remains soluble even after centrifugation. B. To generate Akita-Myc in the membrane extract, HEK 293T cells expressing Akita-Myc were treated with digitonin and centrifuged to generate two fractions. The supernatant fraction contains cytosol proteins (cytosol) and the pellet fraction harbors membranes including the ER (membrane). Both fractions were immunoblotted with the indicated antibodies. C. Coomassie gel of FLAG-tagged Grp170, BiP, G41L Grp170, and BSA. D. (1st – 4th panels) Membrane fraction containing Akita-Myc was incubated with the indicated protein for the indicated time. The samples were centrifuged, and the supernatant material separated from the pellet; the pellet material was resuspended in a sample buffer containing 2% SDS. Both the supernatant and pellet material were analyzed by SDS-PAGE and immunoblotted with an antibody against Myc. (5th panel) Membrane extract containing WT proinsulin-Myc was incubated with BSA and processed as above. The right graph represents the percentage of Akita (or WT proinsulin)-Myc in the pellet fraction compared to the total Akita-Myc signal at each time point from both the soluble and pellet fractions. Data represented the mean ± SD (n=3). The black line indicates that an intervening lane from the same blot has been excised.
