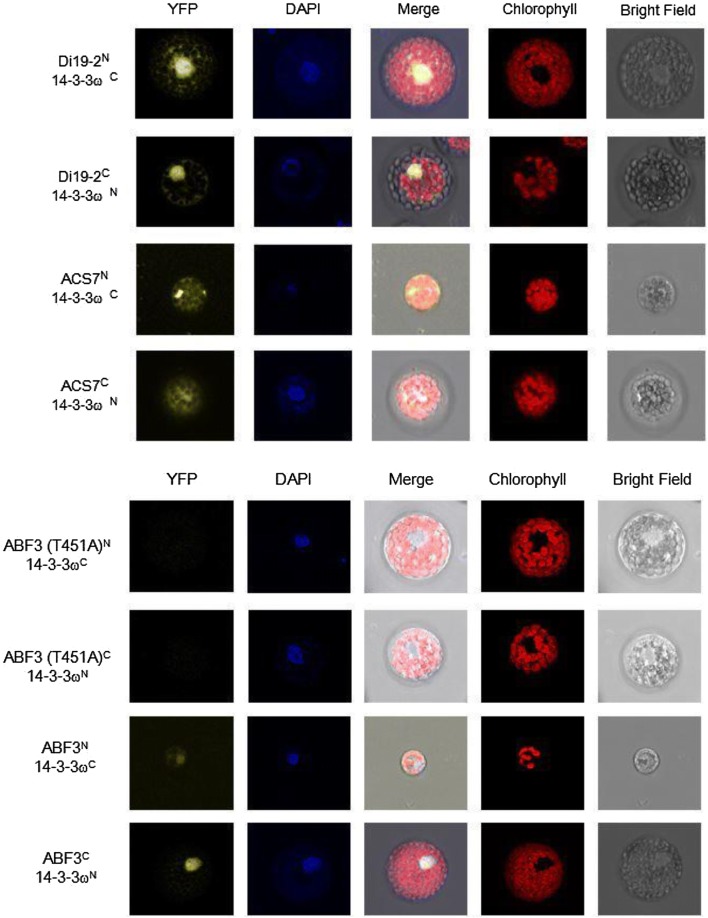
Fig. 4.
ABF3 and 14-3-3ω protein–protein interactions are revealed using bimolecular fluorescence complementation (BiFC). The 14-3-3ω, Di19-2, ACS7, ABF3 proteins were paired with empty vectors used as negative controls. The interaction between YFPN-Di19-2 and YFPC-14-3-3ω/YFPC-Di19-2 and YFPN-14-3-3ω/YFPN-ACS7 and YFPC-14-3-3ω/YFPC-ACS7 and YFPN-14-3-3ω was localized to the nucleus and cytosol as shown in the confocal microscopy images. DAPI was used to stain the nucleus. ACS7 interacts with 14-3-3, and therefore, was introduced as a positive control (Huang et al. 2013). BiFC experiments showed an interaction between ABF3 and 14-3-3ω in Arabidopsis protoplasts. Point mutations of ABF3 (T451A) abolished its interaction with 14-3-3ω. DAPI was used to stain the nucleus