Abstract
Neonatal ethanol exposure during the third trimester equivalent of human pregnancy in the rat significantly impairs hippocampal and prefrontal neurobehavioral functioning. Postnatal day [PD] 4-9 ethanol exposure in rats disrupts long-term context memory formation, resulting in abolished post-shock and retention test freezing in a variant of contextual fear conditioning called the Context Preexposure Facilitation Effect (CPFE). This behavioral impairment is accompanied by disrupted medial prefrontal, but not dorsal hippocampal expression of the immediate early genes (IEGs) c-Fos, Arc, Egr-1, and Npas4 [1]. The current experiment examined if systemic administration of the acetylcholinesterase inhibitor physostigmine (PHY) prior to context learning would rescue prefrontal IEG expression and freezing in the CPFE. From PD4-9, Long-Evans rats received oral intubation of ethanol (EtOH; 5.25g/kg/day) or sham-intubation (SI). Rats received a systemic injection of saline (SAL) or PHY (0.01mg/kg) prior to all three phases (Experiment 1) or just context exposure (Experiment 2) in the CPFE from PD31-33. A subset of rats were sacrificed 30min after context learning to assay changes in IEG expression in the medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC). Administration of PHY prior to all three phases or just context learning rescued both post-shock and retention test freezing in the CPFE in ETOH rats without altering performance in SI rats. ETOH-SAL rats had significantly reduced mPFC but not dHPC expression of c-Fos, Arc, Egr-1, and Npas4. ETOH-PHY treatment rescued mPFC expression of c-Fos in ethanol-exposed rats and increased Arc and Npas4 regardless of dosing condition. While there was no effect of PHY on dHPC or vHPC expression of Arc, Egr-1, or Npas4, this treatment significantly boosted hippocampal expression of c-Fos regardless of ethanol treatment. These findings implicate impaired cholinergic and prefrontal function in cognitive deficits arising from 3rd-trimester equivalent alcohol exposure.
Keywords: cholinergic system, acetylcholine, prefrontal cortex, neonatal ethanol exposure, FASD, context fear, immediate early genes
1. Introduction
Fetal alcohol spectrum disorders (FASDs) represent a spectrum of physical and neurobehavioral impairments caused by gestational alcohol exposure in humans [2]. Alcohol acts as a teratogen in the developing nervous system, causing widespread decreases in brain volume, cortical thickness, neural activity, and altered functional connectivity [3-9]. Gestational exposure alters the normal development of structures such as the basal ganglia, cerebellum, hippocampus, and prefrontal cortex [3,6,7,9,10], resulting in disruptions in learning and memory engaging these structures in exposed children [8,11-20]. Animal models of FASD have proven instrumental in isolating the mechanisms underlying neurobehavioral impairments because of the ability to manipulate alcohol exposure window, pattern, and dosage across discrete phases of development [21]. Rats undergoing binge-like alcohol exposure during the third-trimester equivalent of human pregnancy (e.g., from postnatal day [PD] 4-9, 7-9, or 2-10) show similar neurobiological and behavioral impairments as in the human condition [2,21-24]. This exposure results in altered glutamatergic and cholinergic molecular signaling in the dorsal hippocampus (dHPC) that cannot fully be attributed to cell loss in rats [1,23,25-29]. In contrast, while there is no lasting prefrontal cell loss, neonatal ethanol exposure alters dendritic complexity, neurophysiological properties, and gene expression in the medial prefrontal cortex (mPFC) in rats [1,23,28,30-33].
Recent research in both animal models and humans has strongly suggested a link between acute and chronic alterations in cholinergic signaling in these structures across development as a mechanism underlying ethanol-induced insult [23,34-36]. During the brain growth spurt in rats (i.e., the first 1-2 weeks of life), alcohol interferes with bioavailability of choline and acetylcholine in the brain as well as glial and muscarinic-receptor cell signaling important for neuritogenesis in proliferating pyramidal neurons [37-40]. This exposure persistently alters acetylcholine efflux, muscarinic-receptor cell signaling, and DNA methylation in both the hippocampus and prefrontal cortex [36,41-43]. In animal models, developmental choline supplementation during alcohol exposure reduces ethanol-induced neuroanatomical and molecular insult and, in some cases, rescues behavioral performance in tasks such as the Morris water maze, trace fear conditioning, and eyeblink conditioning [23,44-50]. While more research is needed, these benefits of choline supplementation during gestation are efficacious in the human condition [51-53]. Despite this growing body of work, the effect of augmenting cholinergic system function on neural activity during behavior in ethanol-exposed rats is not well characterized.
Our lab has shown that a variant of Pavlovian contextual fear conditioning, called the Context Preexposure Facilitation Effect (CPFE), is particularly sensitive to the effects of developmental alcohol exposure in rats [1,27,28,54-57]. In the CPFE, learning about the context, acquiring a context-shock association, and retrieval of contextual fear is temporally dissociated across three phases (context preexposure, immediate-shock training, and retention). The CPFE depends on activity and cholinergic muscarinic-receptor cell signaling in the dHPC and mPFC [58-60]. Neonatal ethanol exposure from either PD4-9 or PD7-9 abolishes retention test freezing in the CPFE [28,55-57,61]. We’ve recently shown that PD4-9 ethanol exposure disrupts the consolidation of the context representation during context preexposure, subsequently resulting in abolished post-shock and retention test freezing in the CPFE [1]. This behavioral impairment is accompanied by disrupted medial prefrontal, but not dorsal hippocampal expression of the immediate early genes (IEGs) c-Fos, Arc, Egr-1, and Npas4 during context preexposure. Interestingly, administration of the acetylcholinesterase inhibitor physostigmine (PHY) prior to all three phases rescues retention test freezing in PD7-9 ethanol-exposed rats [62]. The specific phase(s) of the CPFE in which PHY rescues performance and how PHY alters regional IEG expression during the CPFE in ethanol-exposed animals is not known.
The purpose of the current study was to examine the effects of augmenting cholinergic signaling on behavioral performance and medial prefrontal, dorsal hippocampal, and ventral hippocampal IEG expression in ethanol-exposed rats. In Experiment 1, we extended our earlier findings [62] by examining whether or not systemic administration of the acetylcholinesterase inhibitor physostigmine prior to all three phases would rescue freezing in rats receiving PD4-9 ethanol exposure. In Experiment 2, we sought to determine if this same injection prior to just context learning would rescue disrupted prefrontal immediate early gene expression and freezing in the CPFE that we have previously observed [1,28]. To our knowledge, this is the first study to examine the effects of boosting cholinergic function (via PHY) on neural activity during behavior in rats receiving neonatal ethanol exposure during the brain growth spurt.
2. Materials and Methods
2.1. Subjects
Animal husbandry was as described in our previous reports [60,63]. In Experiment 1, there was 43 adolescent (PD31) Long Evans rats (22 females and 21 males), derived from 8 separate litters bred in-house. In Experiment 2, there was 154 PD31 rats (75 females and 79 males), derived from 22 separate litters. The animal housing facility was maintained on a 12:12 h light/dark cycle with lights on at 7:00 am. Litters were culled on PD3 to eight pups (4 males and 4 females when possible), alcohol or sham dosing occurred through PD4-9, and pups were weaned from their mother on PD21 and housed with same-sex littermates. On PD29, rats were individually housed in small clear cages for the remainder of the experiment (until PD33-34). All procedures were approved by the Institutional Animal Care and Use Committee at the University of Delaware following guidelines established by the National Institute of Health.
2.2. Neonatal alcohol dosing (PD4-9, 5.25g/kg/d split into two daily doses)
Neonatal ethanol dosing via intragastric intubation occurred over PD4-9 with methods that have been described previously [1]. Littermates were randomly assigned to receive either ethanol (EtOH group) or sham intubations (SI group), with an equal number of males and females in each litter whenever possible. Same-sex littermates assigned to the same dosing condition (EtOH or SI) were assigned to different experimental groups so that no more than one same-sex littermate was assigned to any particular condition. Briefly, on PD4, pups were separated from their mothers and placed into weigh boats set over a heating pad that provided warmth during the separation. Pups were weighed prior to the first intubation session (occurring daily at 9am ± 1hr). The intubation process involved passing PE10 tubing lubricated with corn oil down the esophagus and into the stomach of the rat pup. Rats in the SI group received intragastric intubations on the same schedule as the EtOH group, and the tube was removed after approximately 6-8 seconds during each scheduled intubation without the infusion of any solution. Rats in the EtOH group were intubated and given a daily dose of 5.25 g/kg of alcohol, [11.9% v/v ethanol (made from 95% ethanol)] in a custom milk formula previously described (Kelly & Lawrence, 2008). This dose was divided into two feedings each day, separated by 2hr. The formula was delivered in a volume of 0.02778 ml/g body weight. A third intubation of the milk formula (containing no ethanol) was administered two hours after the second daily alcohol dosing. After each intubation was completed (<20 minutes per litter), pups were returned as a litter to their mothers. Importantly, although intragastric intubation using these parameters results in a transient reduction in body weight and growth [56,57,64], this reduction is absent by adolescence and thus likely does not contribute to behavioral deficits resulting from exposure [see 64].
2.3. Blood alcohol concentrations (BACs)
On PD4, 90 min following the second alcohol intubation, pups received a small tail-clip and a 20μ1 blood sample was collected using a capillary tube. Blood samples from Group SI were discarded and those from alcohol-exposed pups were saved for further blood alcohol analysis. Blood samples from alcohol-exposed pups were centrifuged, and the plasma was collected and stored at −20°C. Blood alcohol concentrations were determined using an Analox GL5 Analyzer (Analox Instruments, Luneburg, MA) as previously described [55]. Briefly, the rate of oxidation of alcohol in each plasma sample was measured. BACs (expressed in mg/dl) were calculated based on comparisons to known values of an alcohol standard solution.
2.4. Apparatus and stimuli
The apparatus and stimuli used have been described previously [55,60,63]. Briefly, fear conditioning occurred in four clear Plexiglas chambers within a fume hood (Context A – Pre group), with grid floors connected to shock scramblers (Med Associates, Georgia, VT-ENV-414S). The alternate context (Context B – Alt-Pre group) consisted of the same Plexiglas chambers with a convex wire mesh insert that covered the back wall and floor of the chamber and a white paper sleeve that covered the outside walls of the chamber. The unconditioned stimulus (US) was two, 1.5 mA foot-shocks, each 2s in duration, and presented 1s apart immediately upon chamber entry. Videos of each session (preexposure, training, testing) were recorded using Freeze-Frame 3.0 software (Actimetrics, Wilmette IL) with freezing defined as a bout of 0.75 s or longer without a change in video pixilation.
2.5. Context Preexposure Facilitation Effect (CPFE)
The CPFE procedure consisted of three phases (context preexposure, immediate-shock training, retention testing) and took place over the course of three days from PD31 to PD33 as described previously [58-60,63]. In Experiment 1, all rats were preexposed to the same context they were later trained in (Pre group, Context A). In Experiment 2, rats were assigned to this Pre group or an alternate preexposure condition (Alt-Pre group, Context B). Rats in the Pre group received exposure to Context A, the training context, while rats in the Alt-Pre group received exposure to Context B (see section 2.4). Alt-Pre rats serve as non-associative behavioral controls as they demonstrate the immediate-shock deficit (ISD), which reflects an inability to form a context-shock association without prior exposure to Context A [65].
Rats received an intraperitoneal (i.p.) injection of 1ml/kg sterile saline (SAL group) or 0.01mg/ml/kg physostigmine (PHY group) dissolved in saline. This injection occurred 30min prior to all three phases in Experiment 1, and 30min prior to only context preexposure in Experiment 2. During context preexposure on PD31, rats were placed in Context A or B and underwent multiple context preexposure, consisting of one initial 5 min exposure to the chamber, followed by five 1 min exposures, with a 1 min interval between exposures. In Experiment 2, a subset of rats in the Pre group (across both drug conditions) were sacrificed via live decapitation and tissue was collected for RNA extraction and qPCR 30min after context preexposure (see sections 2.6 and 2.7). Alt-Pre rats were not sacrificed as there is no difference between Pre and Alt-Pre gene expression on the preexposure day of the CPFE [63]. In addition, a behaviorally naïve home-cage condition (HC group) was also sacrificed to establish baseline gene expression after drug injection. During immediate-shock training on PD32, rats were carried into the testing room, placed in their respective Context A training chamber, and within 3s, were given two 1.5mA foot-shocks separated by 1s. Rats remained in the training chamber for a 3min post-shock freezing test after foot-shock presentation to measure the immediate acquisition of the context-shock association. During testing on PD33, rats were returned to the same Context A chamber in which they were trained for a 5min retention freezing test. Rats were returned to their home-cages within 1min of the termination of any session (preexposure, training, and testing).
2.6. Brain removal and tissue dissection
In Experiment 2, rats were taken from their home cages 30 min after context preexposure and rapidly decapitated without anesthesia. Brains were removed and bathed in ice-cold saline for about 5-8 sec to increase tissue firmness. Coronal brain slabs (1-1.5 mm) were cut out using a .5mm coronal rat brain matrix. The medial prefrontal cortex (mPFC), dorsal hippocampus (dHPC), and ventral hippocampus (vHPC) were dissected out of the coronal slabs, checking both sides for anterior-posterior boundaries. Consistent with and extending a previous study [63], dissection boundaries were approximately as follows: mPFC, +4.20mm to +2.52mm from bregma; dHPC, 2.16mm to −3.84mm from bregma, vHPC, −4.56 to −6.12 from bregma (using the Paxinos & Watson [2017] rat brain atlas as a guide). Dissected tissue was immediately flash frozen on dry ice and subsequently stored at −80 °C until the time of analysis.
2.7. Quantitative Real-time PCR
The quantitative real-time PCR procedure used has been described previously [63,66]. RNA was extracted from frozen tissue samples using TRIzol Reagent (Cat. No. 15596018, Invitrogen). Genomic DNA was eliminated and cDNA was synthesized from extracted RNA (1000ng/μL) using the QuantiTect® Reverse Transcription Kit (Cat. No. 205314, Qiagen). Relative gene expression was quantified by real-time PCR using the GREEN FASTMIX PERFECTA-SYBR Kit (Cat. No. 101414-270, Quantabio) in 10μL reactions on a CFX96Touch real time PCR machine. Expression of Egr-1 was analyzed using a QuantiTect® Primer Assay (Cat. No. QT00182896, Qiagen) and diluted according to protocol. All other primers were ordered through Integrated DNA Technologies and diluted to a final concentration of 0.13 μM (18s, Arc, c-Fos, and Npas-4). The gene 18s is a ribosomal housekeeping gene and was used as a control for all experimental groups as it did not differ significantly across any groups or manipulations. Samples were numbered, blinded to treatment group and run in duplicate on real-time PCR plates. For each reaction, the average quantitative threshold amplification cycle number (Cq) value was determined from each duplicate, and the 2−ΔΔCq method was used to calculate the relative gene expression for each gene relative to 18s.
2.8. Data analysis and statistics
2.8.1. Analysis of body weight
Neonatal body weight was analyzed with repeated measures ANOVA with a between-subjects factor of dosing condition (SI vs. EtOH) and the within-subjects factor of age (PD4 vs. PD9). There were no main effects or interactions involving sex in the PD4 and PD9 weights (ps > .05) so the data were collapsed across this variable at these ages. Body weight at PD31 was analyzed with a 2 (Sex; male vs. female) × 2 (Dosing condition; SI vs. EtOH) factorial ANOVA. Post-hoc contrasts were performed with Newman–Keuls tests.
2.8.2. Analysis of behavioral data
Behavioral data processing procedures have been described previously [58,63]. A human observer blind to the experimental groups verified the freezing threshold setting with Freeze View 3.0 (Actimetrics, Wilmette IL). The software program computes a “motion index” that was adjusted to set a freezing threshold separately for each animal (per software instructions) by a blind observer who verified from the video record whether or not small movements were scored as freezing. Once set, the threshold did not change during a session. We have validated this procedure against other scoring methods (e.g., hand scoring of video records by two blind observers). Freezing behavior was scored as the total percent time spent freezing longer than .75s bins (defined as the cessation of all movement except breathing) in each respective session bin (context exposure, post-shock freezing, and a 24 h retention test).
The data were imported into STATISTICA 64 data analysis software and freezing behavior was analyzed by ANOVA. There were no main effects or interactions involving sex on freezing behavior (ps > .05), so the data were collapsed across this variable. In Experiment 1, freezing data were analyzed using 2 (Dosing condition; SI vs. EtOH) × 2 (Drug condition; SAL vs. PHY) × 2 (within subjects; phase of testing; Post-shock vs. Retention) repeated measures ANOVA. In Experiment 2, consistent with previous reports [59,60], to reduce animal usage, the Alt-Pre group was pooled across drug but not dosing condition (Pooled-Alt-Pre group) as there was no significant difference between the two drug groups and they froze at uniformly low levels (p > .34). Therefore, freezing data were analyzed using 2 (Dosing; SI vs. EtOH) × 3 (Condition; SAL vs. PHY vs. Pooled-Alt-Pre) × 2 (within subjects; phase of testing; Post-shock vs. Retention) repeated measures ANOVA. Post-hoc contrasts were performed with Newman–Keuls tests. Rats were excluded from analysis as an outlier if they had a score of ± 1.96 standard deviations from the group mean, however, the Z-score of removed outliers averaged ± 3.91 (± 0.97 SEM). We remove outliers in this manner in all of our studies without regard to how it affects the experimental outcome. Outliers removed were as follows: Experiment 1 [(Post-shock: SI-PHY=1, SI-SAL=1)] and Experiment 2 [(Post-shock: EtOH-Pooled-Alt-Pre=1, EtOH-PHY=1, EtOH-SAL=1, SI-PHY=1, SI-SAL=1; Retention: EtOH-Pooled-Alt-Pre=1, SI-Pooled-Alt-Pre=1, EtOH-PHY=2, EtOH-SAL=2)].
2.8.3. Analysis of qPCR data
Relative gene expression for the IEGs c-Fos, Arc, Egr-1, and Npas4 in the mPFC, dHPC, and vHPC was determined (see section 2.7). The relative gene expression value was obtained by normalizing the data to the reference gene (18s) and to the average delta CT of the home-cage control group for each gene. Consistent with previous findings (Heroux et al., 2018; 2019), there were no interactions involving sex (ps > .30), so the data were collapsed across this variable. There was also no difference between the raw data in HC group injected with SAL or PHY, so the HC group was collapsed across drug condition (ps > .35). Gene expression was analyzed using a 2 (Dosing condition; SI vs. EtOH) × 2 (Drug condition; SAL vs. PHY) factorial ANOVA for each gene (c-Fos, Arc, Egr-1, and Npas4) in the mPFC, dHPC, and vHPC. Post-hoc contrasts were performed with Newman–Keuls tests and a Dunnett’s test that contrasted the four experimental groups with the HC control group. The average Z-score of removed outliers was ± 3.14 (± 0.12 SEM; see Table 2).
Table 2.
Final group numbers (n), number of outliers removed (HC, SI-SAL, SI-PHY, EtOH-SAL, EtOH-PHY), and statistical results for all factorial ANOVAs (see F and p values) for each gene (c-Fos, Arc, Egr-1, and Npas4) in each region (mPFC, dHPC, and vHPC) for Experiment 2.
| Medial Prefrontal Cortex (mPFC) | Dorsal Hippocampus (dHPC) | Ventral Hippocampus (vHPC) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | Final n | Outliers | F | p | Final n | Outliers | F | p | Final n | Outliers | |
| Genes | (HC, SI-SAL, SI-PHY, EtOH-SAL, EtOH-PHY) | (HC, SI-SAL, SI-PHY, EtOH-SAL, EtOH-PHY) | (HC, SI-SAL, SI-PHY, EtOH-SAL, EtOH-PHY) | |||||||||
| c-Fos | ||||||||||||
| Dosing | 4.53 | < .05 | 0.58 | > .45 | 0.73 | > .39 | ||||||
| Drug | 7.63 | < .01 | 23, 11, 8, 12, 12 | 1, 1, 1, 0, 0 | 21.59 | < .01 | 21, 10, 10, 11, 11 | 2, 1, 1, 0, 0 | 1.53 | > .23 | 22, 10, 10, 11, 9 | 2, 1, 1, 1, 0 |
| Dosing × Drug | 7.56 | < .01 | 0.12 | > .70 | 4.37 | < .05 | ||||||
| Arc | ||||||||||||
| Dosing | 20.26 | < .01 | 0.11 | > .74 | 0.01 | > .90 | ||||||
| Drug | 18.13 | < .01 | 22, 11, 9, 10, 11 | 2, 1, 0, 2, 1 | 0.1 | > .74 | 21, 10, 10, 10, 10 | 2, 1, 1, 1, 1 | 1.55 | >.22 | 22, 10, 10, 11, 10 | 2, 1, 1, 1, 1 |
| Dosing × Drug | 0.04 | > .80 | 0.03 | > .87 | 1.71 | > .19 | ||||||
| Egr-1 | ||||||||||||
| Dosing | 12.03 | < .01 | 0.54 | > .45 | 0.14 | > .70 | ||||||
| Drug | 2.94 | > .10 | 22, 11, 9, 11, 10 | 2, 1, 0, 1, 2 | 2.83 | > .10 | 20, 11, 10, 10, 10 | 1, 1, 1, 1, 1 | 1.85 | > .18 | 22, 10, 10, 11, 10 | 2, 1, 1, 1, 1 |
| Dosing × Drug | 2.51 | > .12 | 2.75 | > .10 | 0.08 | > .77 | ||||||
| Npas4 | ||||||||||||
| Dosing | 19.2 | < .01 | 0.22 | > .64 | 0.35 | > .56 | ||||||
| Drug | 9.55 | < .01 | 23, 12, 9, 11, 11 | 1, 0, 0, 1, 1 | 0.49 | > .48 | 21, 11, 11, 11, 11 | 2, 0, 0, 1, 0 | 0.07 | > .79 | 22, 10, 10, 12, 10 | 2, 1, 1, 0, 1 |
| Dosing × Drug | 0.49 | > .48 | 0.01 | > .90 | 0.43 | > .50 | ||||||
3. Results
3.1. Body Weights and BACs (both experiments)
Body weight averages for sham-intubated and alcohol-exposed rats at PD4 and PD9 appear in Table 1. Both the SI and EtOH groups gained substantial weight during the dosing period (PD4-PD9) up until the age of testing (PD31). A 2 (Dosing; SI vs. EtOH) × 2 (Age; PD4 vs. PD9) repeated measures ANOVA revealed significant main effects of Dosing condition [F(1, 195) = 67.59, p < .001], Age [F(1, 195) = 7213.30, p < .001], as well as a Dosing × Age interaction [F(1, 195) = 316.35, p < .001]. Newman-Keuls tests revealed no difference between group weights on PD4 (p > .70), but on PD9, EtOH rats weighed about 15% less than SI rats (ps < .001). Transient growth retardation in ethanol treated rats over this dosing period has been reported previously (Murawski et al., 2012; Hamilton et al., 2011; Heroux et al., 2019). Ethanol did not alter body weight at the time of behavioral testing (Table 1). A 2 (Dosing; SI vs. EtOH) × 2 (Sex; male vs. female) factorial ANOVA performed on PD31 body weights revealed a significant main effect of Sex [F(1, 193) = 56.33, p < .001] but not Dosing condition [F(1, 193) = 2.31, p > .13], with no interaction between these two variables [F(1, 193) = 0.00, p > .96]. Females had lower body weights compared to males at PD31 regardless of dosing condition (see Table 1). Finally, the average BAC value taken from the blood samples of the EtOH group in each experiment can be seen in Table 1.
Table 1.
Body weights and BACs for Experiment 1 and 2. Average body weights (in grams ± SE) are given from the SI and EtOH groups at the first and last day of the dosing period (PD4 and PD9, respectively) and the first day of behavioral training (PD31). BACs (in mg/dl ± SE) were taken from blood samples collected on PD4 from the EtOH group. * indicates a significant difference between the SI and EtOH groups.
| Experiment | Dose | n = | Body weight (Grams ± SEM) | PD4 BACs (mg/dl) | |||
|---|---|---|---|---|---|---|---|
| PD4 | PD9 | PD31 (males) | PD31 (females) | ||||
| Experiment 1 | SI | 23 | 12.1 ± 0.27 | 20.8 ± 0.36 | 115.42 ± 2.57 | 108.55 ± 2.87 | N/A |
| EtOH | 20 | 12.06 ± 0.26 | 17.68 ± 0.46* | 110.55 ± 3.24 | 101.27 ± 1.69 | 389.33 ± 4.78 | |
| Experiment 2 | SI | 77 | 11.49 ± 0.14 | 21.23 ± 0.21 | 116.17 ± 1.46 | 106.16 ± 1.57 | N/A |
| EtOH | 77 | 11.42 ± 0.15 | 17.79 ± 0.20* | 115 ± 1.21 | 105.76 ± 1.39 | 407.80 ± 2.86 | |
| Experiment 1 and 2 | SI | 100 | 11.63 ± 0.16 | 21.13 ± 0.18 | 116 ± 1.26 | 106.70 ± 1.37 | N/A |
| Collapsed | EtOH | 97 | 11.56 ± 0.14 | 17.77 ± 0.19* | 114.17 ± 1.16 | 104.75 ± 1.16 | 403.99 ± 2.58 |
3.2. Experiment 1: Systemic administration of physostigmine prior to every phase rescues the CPFE in EtOH-exposed rats
The purpose of Experiment 1 was to determine whether impaired post-shock and retention test freezing in ethanol-exposed rats could be rescued by systemic administration of the acetylcholinesterase inhibitor physostigmine prior to each phase of the CPFE. The behavioral procedure and results for Experiment 1 can be seen in Figure 1A-C. A 2 (Dosing: EtOH vs. SI) × 2 (Drug: SAL vs. PHY) × Phase (Post-shock vs. Retention) between-within factorial design assessed rats exposed only to Context A (Pre, see Section 2.5). We predicted that alcohol would impair freezing in SAL- but not PHY-treated rats.
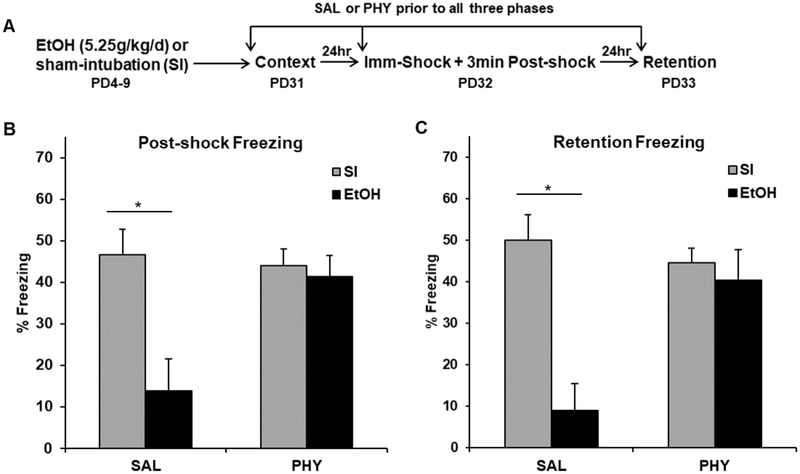
Figure 1.
Behavioral design (A) and mean percent freezing (± SEM) for the 3min post-shock (B) or 5min retention (C) freezing tests in Experiment 1. (A) Rats were given alcohol (EtOH) or sham-intubation (SI) from PD4-9, and given physostigmine (PHY) or saline (SAL) prior to each phase of the CPFE procedure occurring from PD31-33. (B-C) SAL-treated EtOH group rats showed abolished post-shock and retention test freezing compared to SI rats regardless of drug treatment. PHY treatment prior to each phase of the CPFE restored freezing in EtOH rats compared to their SAL-treated counterparts. * indicates p < .05
Analyses for Experiment 1 were run on 41 rats distributed across the following groups: SI-SAL-Pre (n=11), SI-PHY-Pre (n=11), EtOH-SAL-Pre (n=9), and EtOH-PHY-PRE (n=10). ANOVA revealed a significant main effect of Dosing [F(1, 35) = 23.91, p < .001], Drug [F(1, 35) = 8.40, p < .01], and a significant Dosing × Drug interaction [F(1, 35) = 18.23, p < .001]. There was no main effect or any interactions involving the repeated measure of Phase (ps > .45). EtOH-SAL rats showed abolished post-shock and retention test freezing relative to SI-SAL rats (p < .01). PHY treatment eliminated this effect by restoring freezing in the EtOH-PHY group to control levels. Importantly, freezing levels during the preexposure session were uniformly low across experimental conditions; SI and EtOH animals freezing levels were 2.53 ± 0.54 SEM and 1.41 ± 0.21 SEM, respectively. These results demonstrate that systemic PHY administration prior to all three phases of the CPFE rescues impaired context conditioning in ethanol-exposed rats.
3.3. Experiment 2: Systemic administration of physostigmine prior to context preexposure rescues the CPFE and boosts IEG expression in EtOH-exposed rats
3.3.1. Behavioral results
The purpose of Experiment 2 was to examine the effects of physostigmine given only prior to context preexposure on IEG expression and impaired freezing in ethanol-exposed rats. The behavioral procedure and results for Experiment 2 can be seen in Figure 2A-C. Freezing behavior was assessed with a 2 (Dosing: EtOH vs. SI) × 3 (Condition: SAL vs. PHY vs. Pooled-Alt-Pre) × 2 (Phase of testing: Post-shock vs. Retention) between-within factorial design. We predicted that alcohol would impair freezing in SAL- but not PHY-treated rats, with no difference in freezing between EtOH-SAL rats and the non-associative Alt-Pre control group pooled across drug (Pooled-Alt-Pre; see section 2.5).
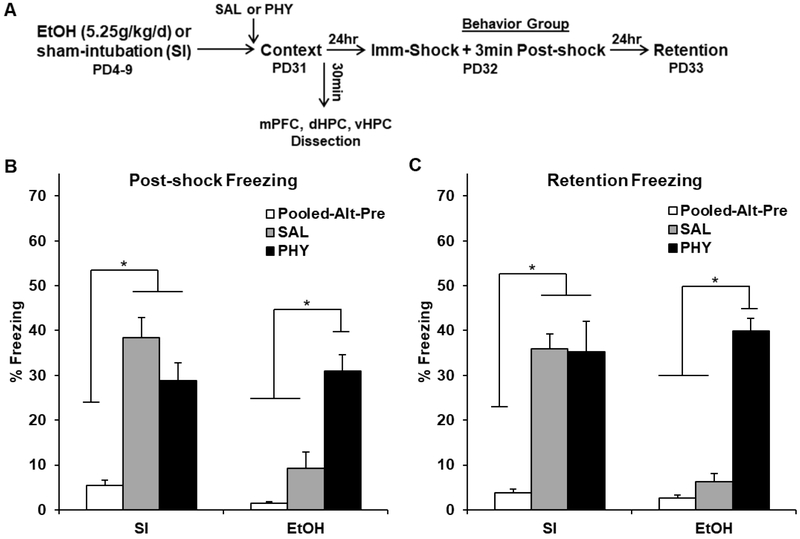
Figure 2.
Behavioral design (A) and mean percent freezing (± SEM) for the 3min post-shock (B) or 5min retention (C) freezing tests in Experiment 2. (A) Rats were given alcohol (EtOH) or sham-intubation (SI) from PD4-9, and given physostigmine (PHY) only prior to context preexposure in the CPFE. (B-C) Freezing above the non-associative Pooled-Alt-Pre control group was present in SI rats regardless of drug, but was only present in EtOH rats given PHY but not SAL. * indicates p < .05
Analyses for Experiment 2 were run on 76 rats distributed across the following groups: SI-Pooled-Alt-Pre (n=14), SI-SAL-Pre (n=13), SI-PHY-Pre (n=12), EtOH-Pooled-Alt-Pre (n=12), EtOH-SAL-Pre (n=12), and EtOH-PHY-PRE (n=13). ANOVA revealed a significant main effect of Dosing [F(1, 63) = 16.58, p < .001], Condition [F(2, 63) = 64.56, p < .001], and a significant Dosing × Condition interaction [F(2, 63) = 21.82, p < .001]. There was no main effect or any interactions involving the repeated measure of Phase (ps > .05). Freezing above the non-associative Pooled-Alt-Pre control group was present in SI rats regardless of drug, but was only present in EtOH rats given PHY (ps < .01). Consistent with Experiment 1, SI and EtOH freezing levels during the preexposure were 2.91 ± 0.75 SEM and 3.23 ± 0.51 SEM, respectively. These results demonstrate that PHY treatment prior to context learning rescues abolished freezing in the CPFE.
3.3.1. IEG results
Littermates of the behavior group were sacrificed 30 min after context exposure on the preexposure day of the CPFE (see sections 2.6 and 2.7). The IEG results can be seen in Figure 3A-C. Gene expression was analyzed using a 2 (Dosing condition; SI vs. EtOH) × 2 (Drug condition; SAL vs. PHY) factorial ANOVA for each gene (c-Fos, Arc, Egr-1, and Npas4) in the mPFC, dHPC, and vHPC. Post-hoc contrasts were performed with Newman–Keuls tests and a Dunnett’s test that contrasted the four experimental groups with the HC control group. The number of outliers removed in each sampling condition can be found in Table 2.
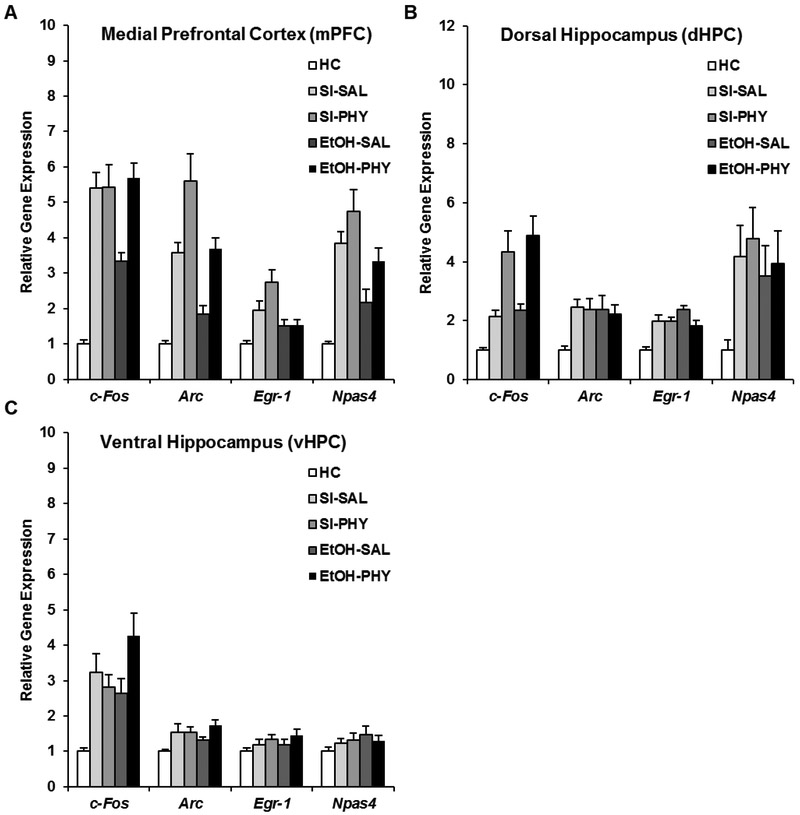
Figure 3.
mRNA expression of c-Fos, Arc, Egr-1, and Npas4 in the mPFC (A), dHPC (B), or vHPC (C) in SI and EtOH rats treated with SAL or PHY sacrificed 30min after context exposure in the CPFE. (A) The EtOH-SAL group showed significantly reduced mRNA expression of every IEG compared to Group SI-SAL. PHY treatment rescued c-Fos expression in the mPFC of EtOH rats, but elevated Arc and npas4 regardless of ethanol treatment. (B) PHY treatment elevated c-Fos expression in the dHPC in both SI and EtOH rats. (C) PHY treatment elevated c-Fos expression in the vHPC in EtOH rats.
In the mPFC (Fig. 3A), the EtOH-SAL group showed significantly reduced mRNA expression of the IEGs c-Fos, Arc, and Npas4 compared to Group SI-SAL (p < .001). Interestingly, PHY administration in EtOH rats specifically rescued prefrontal expression of c-Fos (i.e., the increase occurred in EtOH-PHY but not SI-PHY rats; ps < .01). Both SI and EtOH rats given PHY showed significantly elevated expression of the IEGs Arc and Npas4 above their respective saline-treated groups (ps < .01), indicating a failure of PHY to reverse ethanol effects on these IEGs. While PHY treatment did raise prefrontal egr-1 expression in SI rats (p < .05), there was no difference in expression between the EtOH groups (ps > .20). Dunnett’s tests revealed that every group was significantly elevated above HC levels (ps < .01) with the exception of EtOH-SAL for Arc; and EtOH-SAL and EtOH-PHY for egr-1 expression (p > .05).
In the dHPC (Fig. 3B), no main of interaction effects involving alcohol (dosing) were found for any IEG and a main effect of drug was found only for c-Fos (Table 2). The EtOH-PHY and SI-PHY groups showed significantly higher c-Fos expression than their saline-treated counterparts (p < .01), with no difference between the two groups (p > .40). Dunnett’s tests revealed that SI and EtOH rats given either drug had significantly elevated expression of all IEGs above HC levels. Finally, in the vHPC (Fig. 3C), there were no main or interaction effects of dosing or drug (Table 2). While Dunnett’s tests revealed that all four treated groups had significantly higher expression of c-Fos and Arc than HC levels (ps < .05), there was no elevation above HC in vHPC egr-1 and Npas4 (ps > .10).
Taken together, the results of Experiment 2 demonstrate that systemic PHY administration prior to context preexposure in the CPFE rescues context freezing in ethanol-exposed rats. A similar effect on IEG expression was found only for c-Fos in mPFC. PHY administration increased prefrontal Arc and Npas4 expression, and dHPC c-Fos expression regardless of alcohol treatment. Consistent with previous findings (Heroux et al., 2018) alcohol impaired IEG expression only in mPFC.
4. Discussion
The current set of experiments examined the effects of systemic administration of the acetylcholinesterase inhibitor physostigmine on disruptions in contextual fear conditioning and regional immediate-early gene expression in adolescent rats receiving neonatal ethanol exposure during the brain growth spurt. Consistent with our prior reports [1,27,55,56], high binge-like doses of ethanol given over PD4-9 abolished both post-shock and retention test freezing in the CPFE. This behavioral disruption in ethanol-exposed rats was rescued by PHY administration prior to all three phases (Experiment 1) or just prior to context preexposure (Experiment 2), with no effects of PHY in sham-intubated rats. Furthermore, PHY treatment prior to context learning selectively rescued ethanol-induced disruptions in prefrontal expression of c-Fos but not Npas4 or Arc, or Egr-1. Prefrontal expression of Npas4 and Arc was non-specifically boosted by PHY in both ethanol-exposed and sham-intubated rats, whereas PHY increased Egr-1 in sham but not ethanol-exposed rats. Hippocampal IEG expression was not impaired by alcohol or altered by PHY, except for increased c-Fos expression in dHPC regardless of alcohol exposure. Taken together, augmenting cholinergic signaling rescues neonatal-alcohol induced impairment of configural learning, memory, and prefrontal gene expression.
The present study informs the psychological and neural mechanisms through which neonatal alcohol impairs cognition. The CPFE is a variant of contextual fear conditioning in which learning about the context, acquiring a context-shock association, and retention of contextual fear is separated across three days. The CPFE depends on the encoding of contextual cues on the preexposure day that are subsequently consolidated into a conjunctive context representation [67,68]. During training, pattern completion allows this retrieved conjunctive representation to be associated with immediate foot-shock (i.e. occurring less than 3s upon chamber entry) to yield context fear learning [67]. Rats preexposed to an alternate context are unable to form a context-shock association because of insufficient exposure to the training context [69]. The CPFE develops between PD17 and PD24 in the rat, after which it depends on activity and cholinergic muscarinic-receptor cell signaling in the dHPC and mPFC during all three phases [58-60,68,70]. While impairment of the CPFE is robust across dosing scenarios, neonatal ethanol exposure has no effect on hippocampal-dependent single-trial standard contextual fear conditioning, in which learning about the context and acquiring a context-shock association occur within the same session [1,71,72]. Neonatal ethanol exposure impairs the consolidation of the conjunctive context representation on the preexposure day of the CPFE [1,61], resulting in abolished post-shock and retention test freezing [see Experiment 1 and 2]. Moreover, ethanol-exposed rats have significantly reduced prefrontal but not hippocampal immediate early gene expression during context learning in the CPFE [1,13; see Experiment 2]. In summary, neonatal alcohol impairs incidental context learning or consolidation through a mechanism that may involve reduced prefrontal activity or plasticity.
In the current study, systemic administration of physostigmine prior to all three phases or just prior to context preexposure rescued post-shock and retention test freezing deficits in ethanol-exposed rats. This extends our prior finding that PHY treatment prior to all three phases rescues retention freezing in PD7-9 ethanol-exposed rats [62]. In both cases, the behavioral rescue was specific to ethanol-exposed rats, without any non-specific boost in performance in sham-intubated rats. In addition to rescuing behavioral performance, PHY treatment rescued prefrontal c-Fos expression in ethanol-exposed rats while boosting prefrontal Arc and Npas4 and hippocampal c-Fos expression in both dosing groups. Taken together, these results further support our conclusion that impaired context learning or consolidation by PD4-9 ethanol exposure reflects impaired prefrontal activity or plasticity involving cholinergic signaling.
Developmental alcohol exposure during the brain growth spurt significantly impairs neurobehavioral maturation of the hippocampus and prefrontal cortex in rats (see [1] for extended discussion). Ethanol exposure during the third trimester equivalent of human pregnancy (i.e., PD4-6, PD7-9, PD4-9, or PD2-10) decreases hippocampal CA1 pyramidal cell counts but has no lasting effect on prefrontal cell number in rats [25-27,29,30,54,73]. In addition to cell loss, ethanol exposure disrupts cholinergic and glutamatergic cell signaling, gene expression, and neuroplasticity in the hippocampus [23,27,29,36,74-76]. Although the current study failed to find any alcohol-induced disruption in hippocampal IEG expression during context learning, this does not rule out the possibility that hippocampal dysfunction could contribute to the current findings (see [1] for extended discussion). It’s possible that the current study did not observe disrupted hippocampal IEG expression due to sampling the entire dHPC (with PCR) vs. distinct sub-regions (with in situ hybridization or stereology), examining one time-point, and differences in dosing window or cell-type specificity. In contrast to the hippocampus, neonatal ethanol exposure results in altered gene expression, dendritic complexity, voltage-gated Ca2+ channel activity, and increased DNA methylation in the prefrontal cortex [1,23,28,30-32,77]. Importantly, ethanol-induced disruptions in behavioral performance cannot be fully accounted for by hippocampal cell loss [26,27]. For example, rescuing hippocampal CA1 cell loss via Vitamin E supplementation after PD7-9 ethanol exposure does not normalize performance in the Morris Water Maze (MWM) in rats [26]. Indeed, that PHY treatment prior to only context preexposure rescues the CPFE suggests that the lasting effects of ethanol on behavior are likely due to altered connectivity or molecular signaling of neurons rather than being solely due to developmental injury or loss of neurons themselves. Moreover, our data suggest that reduced activity or plasticity in the prefrontal cortex significantly contributes to ethanol-induced disruptions in behavior that have traditionally been solely attributed to the hippocampus.
Emerging evidence in both human and animal models has demonstrated a link between disrupted cholinergic function across development as a mechanism and potential therapeutic target underlying ethanol-induced insult [see 34 for discussion]. In rats, neonatal ethanol exposure reduces acetylcholine efflux and bioavailability of choline [39,40,43]. Ethanol-induced disruption of muscarinic-receptor cell signaling during development interferes with neuritogenesis and alters muscarinic-receptor cell composition in the hippocampus [35,36,39,41]. Developmental choline supplementation during and after alcohol exposure mitigates many of the neurobiological and behavioral disruptions caused by developmental alcohol exposure. For example, choline reverses increased global DNA methylation in the hippocampus and prefrontal cortex caused by PD2-10 ethanol exposure in rats [23]. Ethanol-induced disruptions in the MWM, fear conditioning, spatial discrimination, working memory, and motor behavior are attenuated by developmental choline supplementation in rats [44-46,48-50,78,79]. Thus far, research has focused on examining the effects of prolonged choline supplementation on ethanol-induced disruptions in behavior later in life. Here, we show that boosting cholinergic signaling via acute treatment with an acetylcholinesterase inhibitor prior to learning attenuates ethanol-induced behavioral disruptions in contextual fear conditioning in adolescent rats [62]. Interestingly, deficits in trace fear conditioning in rats receiving PD4-9 ethanol exposure are also dose-dependently rescued by systemic PHY treatment prior to training [44]. While more research is needed, some mechanisms by which PHY treatment may rescue neurobehavioral deficits by decreasing neuroinflammation, lowering the threshold for LTP induction, and increasing bioavailability of acetylcholine [36,80-82].
The current study supports and extends previous literature examining the neurobiology of contextual fear conditioning and the role of IEGs in learning and memory. We chose to examine the IEGs c-Fos, Arc, Egr-1, and Npas4 because we have recently reported that neonatal ethanol exposure impairs expression of these IEGs during context learning [1,28]. Moreover, context exposure and fear training induces expression of these IEGs in the prefrontal cortex, hippocampus, and amygdala in rats [1,27,83]. These IEGs are also expressed during spatial learning, as well as cued, trace, and contextual fear conditioning across various stages of development [63,83-98]. The IEGs c-Fos, Egr-1, and Npas4 are transcription factors that regulate the expression of other plasticity-associated late-response genes that support long-term memory, whereas Arc encodes a protein that directly regulates dendritic synaptic plasticity [99-101]. While their role is likely universal in supporting memory, the IEGs Arc and Npas4 have been suggested to be important for consolidation of contextual and spatial memories [84,89,91,98,102], and c-Fos and Egr-1 have been studied in relation to the consolidation of fear memories [94,103-108]. Despite emerging evidence supporting a role of the prefrontal circuitry across learning paradigms, especially those relevant to alcohol-induced insult [1,60,88,109-111], examination of the role of prefrontal IEG-expressing neurons in memory has been largely ignored in favor of the hippocampus. Although the mechanism is not known, we show that PHY treatment in ethanol-exposed rats differentially elevates expression of c-Fos, Arc, and Npas4 in the prefrontal cortex and hippocampus. PHY treatment eliminated the alcohol-induced deficit in context fear and in prefrontal c-Fos expression. For other prefrontal IEG expression as well as for dHPC c-Fos expression, PHY treatment elevated IEG expression but did not rescue the alcohol-induced deficit. While the current study cannot fully establish causality between gene expression and behavior, the data suggest a mechanistic role for prefrontal c-Fos expression. Increased acetylcholine bioavailability via systemic PHY treatment elevates c-Fos expression by activating neuronal nicotinic and muscarinic receptors across receptor-populated brain regions, including the prefrontal cortex [112-114]. While there is a clear disruption in dHPC cholinergic receptor function after neonatal alcohol exposure in rats, this disruption likely extends beyond the dHPC and might inform our findings in the mPFC [36]. Given that c-Fos expression is linked to neuronal activity and plasticity necessary for cellular consolidation of long-term memory [99,100], PHY treatment likely recues alcohol-induced deficits by augmenting plasticity-related protein expression. It is also possible that elevated prefrontal Arc and hippocampal c-Fos expression in ETOH-PHY rats contributed to the rescue by exceeding a threshold of expression that was not met in EtOH-SAL rats. Another contributing factor might be IEG expression differences between neuronal sub-regions (e.g., IL vs. PL) and sub-types between dosing conditions, which may contribute to the cholinergic rescue of neurobehavioral effects of alcohol. More research is needed to establish mechanistic roles of cholinergic signaling, regional expression of specific IEGs, and behavioral performance in normally developing and ethanol-exposed rats.
In summary, our findings demonstrate that the acetylcholinesterase inhibitor physostigmine rescues ethanol-induced disruptions in context memory and prefrontal expression of some immediate early genes in adolescent rats. While the current study cannot establish a causal link between IEG expression and behavior, these results suggest that ethanol disrupts behavioral performance by altering activity and/or plasticity induced by cholinergic signaling in the prefrontal cortex during configural learning and memory. Taken together with our recent reports [1,28,62], these findings are important because prefrontal dysfunction is an integral hallmark of FASD in humans, but animal models have thus far largely failed to capture prefrontal dysfunction after third-trimester equivalent exposure. Building upon an emerging body of animal and human research linking alcohol and cholinergic dysfunction, future experiments should characterize the effects of intra-cranial infusions of drugs that augment cholinergic signaling into mPFC or other discrete brain regions on behavioral performance of alcohol-exposed rats across the lifespan.
Highlights.
Neonatal alcohol exposure disrupts context and contextual fear memory in rats
Alcohol exposure disrupts prefrontal but not hippocampal IEG expression
Systemic acetylcholinesterase inhibition rescues behavioral deficits in exposed rats
This treatment specifically reverses deficits in prefrontal c-Fos in alcohol-exposed rats
This treatment nonspecifically elevates prefrontal Arc, Npas4, and hippocampal c-Fos
Acknowledgements
This work is supported by F31AA026503 to NAH and UNIDEL funds to MES. We thank both Dr. Jaclyn Schwarz for generously sharing her lab facilities and Claudia Pinizzotto for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Heroux NA, Robinson-Drummer PA, Kawan M, Rosen JB, Stanton ME, Neonatal ethanol exposure impairs long-term context memory formation and prefrontal immediate early gene expression in adolescent rats, Behav. Brain Res. 359 (2019) 386–395. doi: 10.1016/j.bbr.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murawski NJ, Moore EM, Thomas JD, Riley EP, Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies., Alcohol Res. 37 (2015)97–108. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4476607&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- [3].Moore EM, Migliorini R, Infante MA, Riley EP, Fetal Alcohol Spectrum Disorders: Recent Neuroimaging Findings., Curr. Dev. Disord. Reports. 1 (2014) 161–172. doi: 10.1007/s40474-014-0020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang Y, Roussotte F, Kan E, Sulik KK, Mattson SN, Riley EP, Jones KL, Adnams CM, May PA, O’Connor MJ, Narr KL, Sowell ER, Abnormal cortical thickness alterations in fetal alcohol spectrum disorders and their relationships with facial dysmorphology, Cereb. Cortex. 22 (2012) 1170–1179. doi: 10.1093/cercor/bhr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hendrickson TJ, Mueller BA, Sowell ER, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Wozniak JR, Cortical gyrification is abnormal in children with prenatal alcohol exposure, Neuroimage Clin. 15 (2017) 391–400. doi: 10.1016/j.nicl.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Norman AL, Crocker N, Mattson SN, Riley EP, Neuroimaging and fetal alcohol spectrum disorders, Dev. Disabil. Res. Rev. 15 (2009) 209–217. doi: 10.1002/ddrr.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wozniak JR, Mueller BA, Mattson SN, Coles CD, Kable JA, Jones KL, Boys CJ, Lim KO, Riley EP, Sowell ER, The Cifasd, Functional connectivity abnormalities and associated cognitive deficits in fetal alcohol Spectrum disorders (FASD), Brain Imaging Behav. (2016) 1–14. doi: 10.1007/s11682-016-9624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Infante MA, Moore EM, Bischoff-Grethe A, Tapert SF, Mattson SN, Riley EP, Altered functional connectivity during spatial working memory in children with heavy prenatal alcohol exposure, Alcohol. 64 (2017) 11–21. doi: 10.1016/j.alcohol.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Donald KA, Fouche JP, Roos A, Koen N, Howells FM, Riley EP, Woods RP, Zar HJ, Narr KL, Stein DJ, Alcohol exposure in utero is associated with decreased gray matter volume in neonates, Metab. Brain Dis. 31 (2016) 81–91. doi: 10.1007/s11011-015-9771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Willoughby KA, Sheard ED, Nash K, Rovet J, Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood, J Int Neuropsychol Soc. 14 (2008) 1022–1033. doi:S1355617708081368[pii]\r10.1017/S1355617708081368[doi]. [DOI] [PubMed] [Google Scholar]
- [11].Cheng DT, Jacobson SW, Jacobson JL, Molteno CD, Stanton ME, Desmond JE, Eyeblink classical conditioning in alcoholism and fetal alcohol spectrum disorders, Front. Psychiatry. 6 (2015) 1–7. doi: 10.3389/fpsyt.2015.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE, Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies, Dev. Disabil. Res. Rev. 15 (2009) 176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- [13].Jacobson SW, Stanton ME, Dodge NC, Pienaar M, Fuller DS, Molteno CD, Meintjes EM, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, Impaired Delay and Trace Eyeblink Conditioning in School-Age Children With Fetal Alcohol Syndrome, Alcohol. Clin. Exp. Res. 35 (2011) 250–264. doi: 10.1111/j.1530-0277.2010.01341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, Robinson LK, Khaole N, Jacobson JL, Impaired eyeblink conditioning in children with fetal alcohol syndrome, Alcohol. Clin. Exp. Res. 32 (2008) 365–372. doi: 10.1111/j.1530-0277.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- [15].Kodituwakku PW, Neurocognitive profile in children with fetal alcohol spectrum disorders, Dev. Disabil. Res. Rev. 15 (2009) 218–224. doi: 10.1002/ddrr.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hamilton DA, Kodituwakku P, Sutherland RJ, Savage DD, Children with Fetal Alcohol Syndrome are impaired at place learning but not cued-navigation in a virtual Morris water task, Behav. Brain Res. 143 (2003) 85–94. doi: 10.1016/S0166-4328(03)00028-7. [DOI] [PubMed] [Google Scholar]
- [17].a Uecker L Nadel, Spatial locations gone awry: object and spatial memory deficits in children with fetal alcohol syndrome, Neuropsychologia. 34 (1996) 209–223. doi: 10.1016/0028-3932(95)00096-8. [DOI] [PubMed] [Google Scholar]
- [18].Panczakiewicz AL, Glass L, Coles CD, Kable JA, Sowell ER, Wozniak JR, Jones KL, Riley EP, Mattson SN, Neurobehavioral Deficits Consistent Across Age and Sex in Youth with Prenatal Alcohol Exposure, Alcohol. Clin. Exp. Res. 40 (2016) 1971–1981. doi: 10.1111/acer.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Malisza KL, Allman A-A, Shiloff D, Jakobson L, Longstaffe S, Chudley AE, Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: a functional magnetic resonance imaging study, Pediatr. Res. 58 (2005) 1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- [20].May PA, Chambers CD, Kalberg WO, Zellner J, Feldman H, Buckley D, Kopald D, Hasken JM, Xu R, Honerkamp-Smith G, Taras H, Manning MA, Robinson LK, Adam MP, Abdul-Rahman O, Vaux K, Jewett T, Elliott AJ, Kable JA, Akshoomoff N, Falk D, Arroyo JA, Hereld D, Riley EP, Charness ME, Coles CD, Warren KR, Jones KL, Hoyme HE, Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities, JAMA. 319 (2018) 474. doi: 10.1001/jama.2017.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Patten AR, Fontaine CJ, Christie BR, A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors., Front. Pediatr. 2 (2014) 93. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Driscoll CD, Streissguth AP, Riley EP, Prenatal alcohol exposure: Comparability of effects in humans and animal models, Neurotoxicol. Teratol. 12 (1990) 231–237. doi: 10.1016/0892-0362(90)90094-S. [DOI] [PubMed] [Google Scholar]
- [23].Otero NKH, Thomas JD, Saski CA, Xia X, Kelly SJ, Choline Supplementation and DNA Methylation in the Hippocampus and Prefrontal Cortex of Rats Exposed to Alcohol During Development, Alcohol. Clin. Exp. Res. 36 (2012) 1701–1709. doi: 10.1111/j.1530-0277.2012.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Perkins A, Lehmann C, Lawrence RC, Kelly SJ, Alcohol exposure during development: Impact on the epigenome, Int. J. Dev. Neurosci. 31 (2013) 391–397. doi: 10.1016/j.ijdevneu.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Livy DJ, Miller EK, Maier SE, West JR, Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus, Neurotoxicol. Teratol. 25 (2003) 447–458. doi: 10.1016/S0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- [26].Marino MD, Aksenov MY, Kelly SJ, Vitamin E protects against alcohol-induced cell loss and oxidative stress in the neonatal rat hippocampus, Int. J. Dev. Neurosci. 22 (2004) 363–377. doi: 10.1016/j.ijdevneu.2004.04.005. [DOI] [PubMed] [Google Scholar]
- [27].Murawski NJ, Klintsova AY, Stanton ME, Neonatal alcohol exposure and the hippocampus in developing male rats: Effects on behaviorally induced CA1 c-Fos expression, CA1 pyramidal cell number, and contextual fear conditioning, Neuroscience. 206 (2012) 89–99. doi: 10.1016/j.neuroscience.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jablonski SA, Robinson-Drummer PA, Schreiber WB, Asok A, Rosen JB, Stanton ME, Impairment of the Context Pre-exposure Facilitation Effect in Juvenile Rats by Neonatal Alcohol Exposure is Associated with Decreased Egr-1 mRNA Expression in the Prefrontal Cortex, Behav. Neurosci. 132 (2018) 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tran TD, Kelly SJ, Critical periods for ethanol-induced cell loss in the hippocampal formation, Neurotoxicol. Teratol. 25 (2003) 519–528. doi: 10.1016/S0892-0362(03)00074-6. [DOI] [PubMed] [Google Scholar]
- [30].Hamilton GF, Whitcher LT, Klintsova AY, Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC layer II/III pyramidal neurons, Synapse. 64 (2010) 127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lawrence RC, Otero NKH, Kelly SJ, Selective effects of perinatal ethanol exposure in medial prefrontal cortex and nucleus accumbens, Neurotoxicol. Teratol. 34 (2012) 128–135. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Granato A, Palmer LM, De Giorgio A, Tavian D, Larkum ME, Early Exposure to Alcohol Leads to Permanent Impairment of Dendritic Excitability in Neocortical Pyramidal Neurons, J. Neurosci. 32 (2012) 1377–1382. doi: 10.1523/JNEUROSCI.5520-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Granato A, Di Rocco F, Zumbo A, Toesca A, Giannetti S, Organization of cortico-cortical associative projections in rats exposed to ethanol during early postnatal life, Brain Res. Bull. 60 (2003) 339–344. doi: 10.1016/S0361-9230(03)00052-2. [DOI] [PubMed] [Google Scholar]
- [34].Costa LG, Guizzetti M, Muscarinic cholinergic receptor signal transduction as a potential target for the developmental neurotoxicity of ethanol, Biochem. Pharmacol. 57 (1999) 721–726. doi: 10.1016/S0006-2952(98)00278-0. [DOI] [PubMed] [Google Scholar]
- [35].Wilhelm CJ, Guizzetti M, Fetal Alcohol Spectrum Disorders: An Overview from the Glia Perspective., Front. Integr. Neurosci. 9 (2015) 65. doi: 10.3389/fnint.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Monk BR, Leslie FM, Thomas JD, The effects of perinatal choline supplementation on hippocampal cholinergic development in rats exposed to alcohol during the brain growth spurt, Hippocampus. 22 (2012) 1750–1757. doi: 10.1002/hipo.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guizzetti M, Moore NH, VanDeMark KL, Giordano G, Costa LG, Muscarinic receptor-activated signal transduction pathways involved in the neuritogenic effect of astrocytes in hippocampal neurons, Eur. J. Pharmacol. 659 (2011) 102–107. doi: 10.1016/j.ejphar.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Goeke CM, Roberts ML, Hashimoto JG, Finn DA, Guizzetti M, Neonatal Ethanol and Choline Treatments Alter the Morphology of Developing Rat Hippocampal Pyramidal Neurons in Opposite Directions, Neuroscience. 374 (2018) 13–24. doi: 10.1016/j.neuroscience.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Guizzetti M, Moore NH, Giordano G, VanDeMark KL, Costa LG, Ethanol inhibits neuritogenesis induced by astrocyte muscarinic receptors, Glia. 58 (2010) 1395–1406. doi: 10.1002/glia.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Costa LG, Giordano G, Guizzetti M, Inhibition of cholinergic muscarinic signaling by ethanol: Potential mechanism of developmental neurotoxicity and biological plausibility for the beneficial effects of choline supplementation, Int. J. Alcohol Drug Res. 2 (2013) 17. doi: 10.7895/ijadr.v2i3.72. [DOI] [Google Scholar]
- [41].Kelly SJ, Black AC Jr., West JR, Changes in the muscarinic cholinergic receptors in the hippocampus of rats exposed to ethyl alcohol during the brain growth spurt, J. Pharmacol. Exp. Ther. 249 (1989) 798–804. [PubMed] [Google Scholar]
- [42].Carneiro LMV, Diógenes JPL, Vasconcelos SMM, Aragão GF, Noronha EC, Gomes PB, Viana GSB, Behavioral and neurochemical effects on rat offspring after prenatal exposure to ethanol, Neurotoxicol. Teratol. 27 (2005) 585–592. doi: 10.1016/j.ntt.2005.06.006. [DOI] [PubMed] [Google Scholar]
- [43].Perkins A, Fadel JR, Kelly SJ, The effects of postnatal alcohol exposure and galantamine on the context pre-exposure facilitation effect and acetylcholine efflux using invivo microdialysis, Alcohol. 49 (2015) 193–205. doi: 10.1016/j.alcohol.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hunt PS, Barnet RC, An animal model of fetal alcohol spectrum disorder: Trace conditioning as a window to inform memory deficits and intervention tactics, Physiol. Behav. 148 (2015) 36–44. doi: 10.1016/j.physbeh.2014.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ryan SH, Williams JK, Thomas JD, Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration, Brain Res. 1237 (2008) 91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wagner AF, Hunt PS, Impaired trace fear conditioning following neonatal ethanol: reversal by choline., Behav. Neurosci. 120 (2006) 482–7. doi: 10.1037/0735-7044.120.2.482. [DOI] [PubMed] [Google Scholar]
- [47].Thomas JD, Tran TD, Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development, Hippocampus. 22 (2012) 619–630. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Idrus NM, Breit KR, Thomas JD, Dietary choline levels modify the effects of prenatal alcohol exposure in rats, Neurotoxicol. Teratol. 59 (2017) 43–52. doi: 10.1016/j.ntt.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Thomas JD, Biane JS, O’Bryan KA, O’Neill TM, Dominguez HD, Choline supplementation following third-trimester-equivalent alcohol exposure attenuates behavioral alterations in rats., Behav. Neurosci. 121 (2007) 120–130. doi: 10.1037/0735-7044.121.1.120. [DOI] [PubMed] [Google Scholar]
- [50].Thomas JD, Idrus NM, Monk BR, Dominguez HD, Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats, Birth Defects Res. Part A Clin. Mol. Teratol. 88 (2010) 827–837. doi: 10.1002/bdra.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wozniak JR, Fuglestad AJ, Eckerle JK, Kroupina MG, Miller NC, Boys CJ, Brearley AM, Fink BA, Hoecker HL, Zeisel SH, Georgieff MK, Choline supplementation in children with fetal alcohol spectrum disorders has high feasibility and tolerability, Nutr. Res. 33 (2013) 897–904. doi: 10.1016/j.nutres.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Wozniak JR, A.J. F, J.K. E, B.A. F, H.L. H, C.J. B, J.P. R, M.G. K, N.C. M, A.M. B, S.H. Z, Choline supplementation in children with fetal alcohol spectrum disorders: A randomized, double-blind, placebo-controlled trial, Am. J. Clin. Nutr. 102 (2015) 1113–1125. doi: 10.3945/ajcn.114.099168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jacobson SW, Carter RC, Molteno CD, Stanton ME, Herbert JS, Lindinger NM, Lewis CE, Dodge NC, Hoyme HE, Zeisel SH, Meintjes EM, Duggan CP, Jacobson JL, Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial, Alcohol. Clin. Exp. Res. 42 (2018) 1327–1341. doi: 10.1111/acer.13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hamilton GF, Murawski NJ, St. Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY, Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats, Brain Res. 1412 (2011) 88–101. doi: 10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Murawski NJ, Stanton ME, Variants of contextual fear conditioning are differentially impaired in the juvenile rat by binge ethanol exposure on postnatal days 4–9, Behav. Brain Res. 212 (2010) 133–142. doi: 10.1016/j.bbr.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Murawski NJ, Stanton ME, Effects of dose and period of neonatal alcohol exposure on the context preexposure facilitation effect, Alcohol. Clin. Exp. Res. 35 (2011) 1160–1170. doi: 10.1111/j.1530-0277.2011.01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Jablonski SA, Stanton ME, Neonatal alcohol impairs the context preexposure facilitation effect in juvenile rats: Dose-response and post-training consolidation effects, Alcohol. 48 (2014) 35–42. doi: 10.1016/j.alcohol.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Robinson-Drummer PA, Dokovna LB, Heroux NA, Stanton ME, Cholinergic mechanisms of the context preexposure facilitation effect in adolescent rats., Behav. Neurosci. 130 (2016) 196–205. doi: 10.1037/bne0000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Robinson-Drummer PA, Heroux NA, Stanton ME, Antagonism of muscarinic acetylcholine receptors in medial prefrontal cortex disrupts the context preexposure facilitation effect, Neurobiol. Learn. Mem. 143 (2017) 27–35. doi: 10.1016/j.nlm.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Heroux NA, Robinson-Drummer PA, Sanders HR, Rosen JB, Stanton ME, Differential involvement of the medial prefrontal cortex across variants of contextual fear conditioning, Learn. Mem. 3 (2017) 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Goodfellow MJ, Lindquist DH, Significant long-term, but not short-term, hippocampal-dependent memory impairment in adult rats exposed to alcohol in early postnatal life, Dev. Psychobiol. 56 (2014) 1316–1326. doi: 10.1002/dev.21210. [DOI] [PubMed] [Google Scholar]
- [62].Dokovna LB, Jablonski SA, Stanton ME, Neonatal alcohol exposure impairs contextual fear conditioning in juvenile rats by disrupting cholinergic function, Behav. Brain Res. 248 (2013) 114–120. doi: 10.1016/j.bbr.2013.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Heroux NA, Osborne BF, Miller LA, Kawan M, Buban KN, Rosen JB, Stanton ME, Differential expression of the immediate early genes c-Fos , Arc , Egr-1 , and Npas4 during long-term memory formation in the context preexposure facilitation effect (CPFE), Neurobiol. Learn. Mem. 147 (2018) 128–138. doi: 10.1016/j.nlm.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kelly SJ, Lawrence RC, Intragastric intubation of alcohol during the perinatal period, 2008. doi: 10.1007/978-1-59745-242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Fanselow MS, Factors governing one-trial contextual conditioning, Anim. Learn. Behav. 18 (1990) 264–270. doi: 10.3758/BF03205285. [DOI] [Google Scholar]
- [66].Livak KJ, Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 22DDCT Method, Methods. 25 (2001) 402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [67].Rudy JW, Context representations, context functions, and the parahippocampal-hippocampal system, Learn. Mem. 16 (2009) 573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Jablonski SA, Schiffino FL, Stanton ME, Role of age, post-training consolidation, and conjunctive associations in the ontogeny of the context preexposure facilitation effect, Dev. Psychobiol. 54 (2012) 714–722. doi: 10.1002/dev.20621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fanselow MS, Conditional and unconditional components of post shock freezing., Pavlov.J.Biol.Sci. 15 (1980) 177–182. [DOI] [PubMed] [Google Scholar]
- [70].Schiffino FL, Murawski NJ, Rosen JB, Stanton ME, Ontogeny and neural substrates of the context preexposure facilitation effect, Neurobiol. Learn. Mem. 95 (2011) 190–198. doi: 10.1016/j.nlm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS, Context Fear Learning in the Abssence of the Hippocampus, J. Neurosci. 26 (2006) 5484–5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Zelikowsky M, Bissiere S, Hast TA, Bennett RZ, Abdipranoto A, Vissel B, Fanselow MS, Prefrontal microcircuit underlies contextual learning after hippocampal loss., Proc. Natl. Acad. Sci. U. S. A. 110 (2013) 9938–43. doi: 10.1073/pnas.1301691110/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1301691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bonthius DJ, West JR, Alcohol-Induced Neuronal Loss in Developing Rats: Increased Brain Damage with Binge Exposure, Alcohol. Clin. Exp. Res. 14 (1990) 107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- [74].DuPont CM, Coppola JJ, Kaercher RM, Lindquist DH, Impaired trace fear conditioning and diminished ERK1/2 phosphorylation in the dorsal hippocampus of adult rats administered alcohol as neonates., Behav. Neurosci. 128 (2014) 187–98. doi: 10.1037/a0035989. [DOI] [PubMed] [Google Scholar]
- [75].Goodfellow MJ, Abdulla KA, Lindquist DH, Neonatal Ethanol Exposure Impairs Trace Fear Conditioning and Alters NMDA Receptor Subunit Expression in Adult Male and Female Rats, Alcohol. Clin. Exp. Res. 40 (2016) 309–318. doi: 10.1111/acer.12958. [DOI] [PubMed] [Google Scholar]
- [76].Puglia MP, Valenzuela CF, Repeated third trimester-equivalent ethanol exposure inhibits long-term potentiation in the hippocampal CA1 region of neonatal rats, Alcohol. 44 (2010) 283–290. doi: 10.1016/j.alcohol.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Nagahara AH, Handa RJ, Fetal alcohol exposure alters the induction of immediate early gene mRNA in the rat prefrontal cortex after an alternation task, Alcohol. Clin. Exp. Res. 19 (1995) 1389–1397. doi: 10.1111/j.1530-0277.1995.tb00997.x. [DOI] [PubMed] [Google Scholar]
- [78].Thomas JD, Garrison M, O’Neill TM, Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats, Neurotoxicol. Teratol. 26 (2004) 35–45. doi: 10.1016/j.ntt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- [79].Thomas JD, Abou EJ, Dominguez HD, Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats, Neurotoxicol. Teratol. 31 (2009) 303–311. doi: 10.1016/j.ntt.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kalb A, von Haefen C, Sifringer M, Tegethoff A, Paeschke N, Kostova M, Feldheiser A, Spies CD, Acetylcholinesterase Inhibitors Reduce Neuroinflammation and -Degeneration in the Cortex and Hippocampus of a Surgery Stress Rat Model, PLoS One. 8 (2013) 1–13. doi: 10.1371/journal.pone.0062679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Goodfellow MJ, Shin YJ, Lindquist DH, Mitigation of postnatal ethanol-induced neuroinflammation ameliorates trace fear memory deficits in juvenile rats, Behav. Brain Res. 338 (2018) 28–31. doi: 10.1016/j.bbr.2017.09.047. [DOI] [PubMed] [Google Scholar]
- [82].Pyapali GK, a Turner D, Williams CL, Meck WH, Swartzwelder HS, Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats., J. Neurophysiol. 79 (1998) 1790–6. http://www.ncbi.nlm.nih.gov/pubmed/9535948. [DOI] [PubMed] [Google Scholar]
- [83].Schreiber WB, Asok A, Jablonski SA, Rosen JB, Stanton ME, Egr-1 mRNA expression patterns in the prefrontal cortex, hippocampus, and amygdala during variants of contextual fear conditioning in adolescent rats, Brain Res. 1576 (2014) 63–72. doi: 10.1016/j.brainres.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y, Npas4 Regulates a Transcriptional Program in CA3 Required for Contextual Memory Formation, Science (80-. ). 334 (2011) 1670. doi: 10.1088/0004-637X/736/2/160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Ye L, Allen WE, Thompson KR, Tian Q, Hsueh B, Ramakrishnan C, Wang AC, Jennings JH, Adhikari A, Halpern CH, Witten IB, Barth AL, Luo L, McNab JA, Deisseroth K, Wiring and Molecular Features of Prefrontal Ensembles Representing Distinct Experiences, Cell. 165 (2016) 1776–1788. doi: 10.1016/j.cell.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rosen JB, Fanselow MS, Young SL, Sitcoske M, Maren S, Immediate-early gene expression in the amygdala following footshock stress and contextual fear conditioning, Brain Res. 796 (1998) 132–142. doi: 10.1016/S0006-8993(98)00294-7. [DOI] [PubMed] [Google Scholar]
- [87].Sun X, Lin Y, Npas4: Linking Neuronal Activity to Memory, Trends Neurosci. 39 (2016) 264–275. doi: 10.1016/j.tins.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zelikowsky M, Hersman S, Chawla MK, Barnes CA, Fanselow MS, Neuronal Ensembles in Amygdala, Hippocampus, and Prefrontal Cortex Track Differential Components of Contextual Fear, J. Neurosci. 34 (2014) 8462–8466. doi: 10.1523/JNEUROSCI.3624-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Weng FJ, Garcia RI, Lutzu S, Alviña K, Zhang Y, Dushko M, Ku T, Zemoura K, Rich D, Garcia-Dominguez D, Hung M, Yelhekar TD, Sørensen AT, Xu W, Chung K, Castillo PE, Lin Y, Npas4 Is a Critical Regulator of Learning-Induced Plasticity at Mossy Fiber-CA3 Synapses during Contextual Memory Formation, Neuron. 97 (2018) 1137–1152.e5. doi: 10.1016/j.neuron.2018.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Ploski JE, Monsey MS, Nguyen T, DiLeone RJ, Schafe GE, The neuronal PAS domain protein 4 (Npas4) is required for new and reactivated fear memories, PLoS One. 6 (2011). doi: 10.1371/journal.pone.0023760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA, Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory., J. Neurosci. 20 (2000) 3993–4001. doi:20/11/3993 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Guzowski JF, Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches, Hippocampus. 12 (2002) 86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- [93].Lee JLC, Memory reconsolidation mediates the strengthening of memories by additional learning, Nat. Neurosci. 11 (2008) 1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- [94].Lee JLC, Memory Reconsolidation Mediates the Updating of Hippocampal Memory Content, Front. Behav. Neurosci. 4 (2010) 168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Asok A, Schreiber WB, Jablonski SA, Rosen JB, Stanton ME, Egr-1 increases in the prefrontal cortex following training in the context preexposure facilitation effect (CPFE) paradigm, Neurobiol. Learn. Mem. 106 (2013) 145–153. doi: 10.1016/j.nlm.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Robinson-Drummer PA, Chakraborty T, Heroux NA, Rosen JB, Stanton ME, Age and experience dependent changes in Egr-1 expression during the ontogeny of the context preexposure facilitation effect (CPFE), Neurobiol. Learn. Mem. 150 (2018) 1–12. doi: 10.1016/j.nlm.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Messaoudi E, Kanhema T, Soulé J, Tiron A, Dagyte G, da Silva B, Bramham CR, Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo., J. Neurosci. 27 (2007) 10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Pevzner A, Miyashita T, Schiffman AJ, Guzowski JF, Temporal dynamics of Arc gene induction in hippocampus: Relationship to context memory formation, Neurobiol. Learn. Mem. 97 (2012) 313–320. doi: 10.1016/j.nlm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- [99].Gallo FT, Katche C, Morici JF, Medina JH, Weisstaub NV, Immediate Early Genes, Memory and Psychiatric Disorders: Focus on c-Fos, Egr1 and Arc, Front. Behav. Neurosci. 12 (2018) 1–16. doi: 10.3389/fnbeh.2018.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Minatohara K, Akiyoshi M, Okuno H, Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace, Front. Mol. Neurosci. 8 (2016) 78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Alberini CM, Transcription Factors in Long-Term Memory and Synaptic Plasticity, Physiol. Rev. (2009) 121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Guzowski JF, Setlow B, Wagner EK, McGaugh JL, Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268, J. Neurosci. 21 (2001) 5089–5098. doi:21/14/5089 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Malkani S, Wallace KJ, Donley MP, Rosen JB, An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning, Learn. Mem. (Cold Spring Harb. NY). 11 (2004) 617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Slipczuk L, Katche C, Izquierdo IA, Medina JH, Goldin A, Cammarota M, Bekinschtein P, Delayed wave of c-Fos expression in the dorsal hippocampus involved specifically in persistence of long-term memory storage, Proc. Natl. Acad. Sci. 107 (2009) 349–354. doi: 10.1073/pnas.0912931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S, Optogenetic stimulation of a hippocampal engram activates fear memory recall, Nature. 484 (2012) 381–385. doi: 10.1038/nature11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M, Direct Reactivation of a Coherent Neocortical Memory of Context, Neuron. 84 (2014) 432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Katche C, Goldin A, Gonzalez C, Bekinschtein P, Medina JH, Maintenance of long-term memory storage is dependent on late posttraining Egr-1 expression, Neurobiol. Learn. Mem. 98 (2012) 220–227. doi: 10.1016/j.nlm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- [108].Katche C, Medina JH, Requirement of an Early Activation of BDNF/c-Fos Cascade in the Retrosplenial Cortex for the Persistence of a Long-Lasting Aversive Memory, Cereb. Cortex. 27 (2017) 1060–1067. doi: 10.1093/cercor/bhv284. [DOI] [PubMed] [Google Scholar]
- [109].Gilmartin MR, Balderston NL, Helmstetter FJ, Prefrontal cortical regulation of fear learning, Trends Neurosci. 37 (2014) 445–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Giustino TF, Maren S, The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear., Front. Behav. Neurosci. 9 (2015) 298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ramamoorthi K, Ressler RL, Jin J, Maren S, Nucleus reuniens is required for encoding and retrieving precise, hippocampal-dependent contextual fear memories in rats, J. Neurosci. (2018) 1429–18. doi: 10.1523/JNEUROSCI.1429-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Pongrac JL, Rylett RJ, Molecular Mechanisms Regulating NGF-Mediated Enhancement of Cholinergic Neuronal Phenotype: c-Fos Trans-Activation of the Choline Acetyltransferase Gene, J. Mol. Neurosci. 11 (2003) 79–94. doi: 10.1385/jmn:11:1:79. [DOI] [PubMed] [Google Scholar]
- [113].Kaufer D, Friedman A, Seidman S, Soreq H, Acute stress facilitates long-lasting changes in cholinergic gene expression, Nature. 531 (2016) 373–378. [DOI] [PubMed] [Google Scholar]
- [114].Thomsen MS, Mikkelsen JD, Timmermann DB, Peters D, Hay-Schmidt A, Martens H, Hansen HH, The selective α7 nicotinic acetylcholine receptor agonist A-582941 activates immediate early genes in limbic regions of the forebrain: Differential effects in the juvenile and adult rat, Neuroscience. 154 (2008) 741–753. doi: 10.1016/j.neuroscience.2008.03.083. [DOI] [PubMed] [Google Scholar]